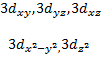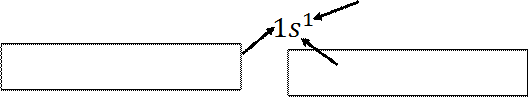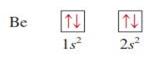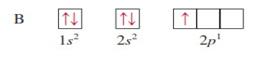Session 1: Introduction to Pharmaceutical
Inorganic Chemistry
Total Session Time: 60 minutes
Prerequisites
None
Learning Tasks
By the end of this session
students are expected to be able to:
Define the term pharmaceutical chemistry
List the branches of pharmaceutical chemistry
Define the term pharmaceutical inorganic
chemistry
Define terminologies used in pharmaceutical
inorganic chemistry Describe
the importance of chemistry in pharmacy
Resources Needed:
Flip charts, marker pens, and masking tape
Black/white board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning Task
|
|
2
|
05 minutes
|
Presentation
|
Definition of
Pharmaceutical Chemistry
|
|
3
|
10minutes
|
Presentation Buzzing
|
Branches of Pharmaceutical
Chemistry
|
|
4
|
05 minutes
|
Presentation
|
Definition of
Pharmaceutical Inorganic Chemistry
|
|
5
|
10 minutes
|
Presentation
|
Terminologies Used in Pharmaceutical
Inorganic Chemistry
|
|
6
|
15 minutes
|
Presentation
|
Importance of Chemistry in
Pharmacy
|
|
7
|
05 minutes
|
Presentation
|
Key Points
|
|
8
|
05 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP1:
Presentation of Session Title and Learning Task (5 minutes)
READ or ASK students to read the learning task and clarify
ASK students if they have any question before continuing.
STEP 2:
Definition of Pharmaceutical Chemistry (5 minutes)
Pharmaceutical chemistry o Refers
to the branch of pharmaceutical science which deals with the composition,
structure, properties, preparation, and analysis of chemical compounds used in
medical diagnosis and treatment
STEP 3:
Branches of Pharmaceutical Chemistry (10 minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What are the branches of pharmaceutical
chemistry?
ALLOW few
pairs to respond and let other pairs to add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY
and SUMMARIZE by using the content
below
|
The following are the branches of
pharmaceutical chemistry:
Pharmaceutical organic chemistry
Study of all substances containing carbon
Pharmaceutical inorganic chemistry
Study of all elements
Analytical chemistry (Pharmaceutical
Analysis)
Study of the composition of substances
Identifies, evaluate and compare components of
matter
Physical pharmaceutical chemistry
Study of theories and experiments that describe
the behavior of chemicals and energy involved
Biochemistry
Study of the chemistry of living organisms
STEP 4:
Definition of Pharmaceutical Inorganic Chemistry (5 minutes)
Pharmaceutical inorganic chemistry o Refers
to the branch of pharmaceutical chemistry dealing with the study of all
elements
Pharmaceutical inorganic chemistry gives the
systemic overview of chemical elements and their compounds together with their
biological and chemical importance in pharmacy
STEP 5:
Terminologies Used in Pharmaceutical Inorganic Chemistry (10 minutes)
Drug
o
Substance having the prophylactic, diagnostic or
therapeutic effects and maybe used in manufacturing of medicinal preparations
Activation energy o The minimum energy required to start a
chemical reaction
Alkali metals
o The
very reactive metals found in Group 1 (I-A) of the periodic table
Alkaline earth metals o Those elements found in Group 2(II-A) of
the periodic table
Binding energy
o A
measure of the strength of the force holding the nucleons together in the nucleus of an atom
Cation o A positively charged ion
Covalent bonds
o Bonds
between atoms formed by sharing two or more valence electrons
Electric dipole
o A
molecule with two regions of opposite charge
Element o A
substance that cannot be split into simpler substances by chemical means
Ground state
o The
lowest stable energy state of a substance or system
Halogens o The
elements that make up Group 17 (VII-A) of the periodic table
Hybridized orbital o The new orbital formed by combination of
atomic orbitals
Hydrogen bond
o A
weak bond between the hydrogen in a polar covalent bond and a neighboring
molecule with a highly electronegative atom
Hydrophilic
o Water-loving
Ion
o
An atom that carries an electric charge due to
the addition or removal of one or more electrons
Ionic bond
o The
bond between ions due to their opposite electrical charges
Isotopes o Atoms
with the same number of protons and electrons but with a different number of
neutrons in the nucleus
Radioactive elements o Elements
capable of emitting alpha, beta, or gamma radiation
Valence o The
highest-energy electrons in an atom, which an atom loses, gains, or shares in
forming a chemical bond
STEP 6:
Importance of Chemistry in Pharmacy (15 minutes)
Exploration of suitable sources of drugs o Chemistry
is involved in all processes of the discovery phase
o
Sources of drug molecules can be natural example
narcotic analgesic, morphine, from Papaversomniferum
(Poppy plant), synthetic, example a popular analgesic and antipyretic,
paracetamol, or semi-synthetic, example semi-synthetic penicillins
o
If a drug molecule has to be purified from a
natural source such as a plant, processes such as extraction, isolation and
identification are used, and all these processes involve chemistry
Exploration of the chemical and the physical
properties of drug o In
the pre-formulation and formulation studies of the drug, chemistry is required
in the determination of structures and the physical properties such as
solubility and pH, of the drug molecules
o
Each type of dosage form to be designed requires
careful study of the physical and chemical properties of drug substances to
achieve a stable and efficacious product
Determination of storage conditions o Chemistry is important in the
exploration of suitable storage condition
o
Example: Drugs with an ester functionality, such
as aspirin, could be quite unstable in the presence of moisture, and should be
kept in a dry and cool place
Determination of packaging material o The concept of chemistry is needed in
the choice of packaging
o
Example amber colored bottle is chosen for drugs
which are sensitive to light
Choice of the appropriate route of
administration o When administered, the action of a drug
inside the body depends on its binding to the appropriate receptor, and its
subsequent metabolic processes, all of which involve complex enzyme driven
biochemical reactions
Study of the biopharmaceutical parameters
(absorption, distribution, metabolism and elimination) so as to understand the
interaction between the drug and the body
Diagnosis and treatment of diseases o Medicines
or drugs that are taken for the treatment of various ailments are chemicals o Various
elements are used for diagnosis (diagnostic radiopharmaceuticals) example
Technetium-99m (99Tc) a common radioisotope used in imaging of
various body organs
o
Various radioactive elements are involved in
treatment of various disorder (therapeutic radiopharmaceuticals) example Sodium
iodide 131 (131I) used for treatment of thyroid cancer
STEP 7: Key
Points (5 minutes)
Pharmaceutical Inorganic chemistry o Refers to the branch of pharmaceutical
chemistry which deals with the study of all elements
o
Branches of pharmaceutical chemistry
includes:
§
Pharmaceutical organic chemistry
§
Pharmaceutical inorganic chemistry
§
Analytical chemistry (Pharmaceutical Analysis)
§
Physical pharmacy and Biochemistry
The importance of chemistry in pharmacy include:
o
Exploration of suitable sources of drugs
o
Exploration of the chemical and the physical
properties of drug o Determination
of storage conditions o Determination
of packaging material o Choice
of the appropriate route of administration o Study of the biopharmaceutical
parameters o Diagnosis
and treatment of diseases
STEP 8:
Evaluation (5 minutes)
What is pharmaceutical chemistry?
What are the branches of pharmaceutical
chemistry?
What is pharmaceutical inorganic chemistry?
What is the importance of chemistry in pharmacy?
References
Chang,
R.,& Overby, J. (2011). General
chemistry: The Essential Concepts (6thed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rd Ed). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S.,& Lutfun, N. (2007).Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21sted.). Baltimore, M.A. , A Wolters
Kluwer Company.
Session 2: Description of an Atom
Total Session Time: 120 minutes + 2 hours of Assignment and
Tutorial
Prerequisites
None
Learning Tasks
By the end of this session
students are expected to be able to:
Define
an atom
Describe
the Daltons atomic theory Describe the
structure of an atom
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
05 minutes
|
Presentation
|
Definition of an Atom
|
|
3
|
20 minutes
|
Presentation
Buzzing
|
Daltons Atomic Theory
|
|
4
|
60 minutes
|
Presentation Small group discussion
|
Structure of an Atom
|
|
5
|
10 minutes
|
Presentation
|
Key Points
|
|
6
|
10 minutes
|
Presentation
|
Evaluation
|
|
7
|
10 minutes
|
Presentation
|
Assignment
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students to read the learning tasks and clarify
ASK students if they have any questions before continuing.
STEP 2:
Definition of an Atom (5 minutes)
Atom
o
Refers to the smallest particle of an element
that can take part in a chemical reaction
o It
is a basic unit of matter
STEP 3:
Daltons Atomic Theory (20 minutes)
|
Activity: Buzzing (10 minutes)
ASK students to pair up and buzz on
the following questions for 5 minutes What
is the Daltons atomic theory?
What
are the shortcoming and amendments of the Daltons atomic theory?
ALLOW few
pairs to respond and let other pairs to add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
Daltons Atomic Theory
All
matter is composed of tiny, indivisible particles called atoms
Atoms
cannot be made or destroyed
All
atoms of one element are identical in weight and atoms of different elements
have different weight
Compounds
are formed by joining the atoms of two or more elements o When forming a compound, the atoms of
elements combine in whole-number ratios, such as 1 to 1, 2 to 1, 3 to 2, and so
on
A
chemical reaction is the rearrangement of atoms
Shortcomings of the Daltons Atomic Theory
Matter
is composed of atoms which are divisible
All
atoms of one element may not be identical in weight
Amendments of the Daltons Atomic Theory
Basing on the Modern Concepts
Atoms are composed of smaller subatomic
particles (protons, neutrons and electrons) thus it can be broken down
Elements
have Isotopes (atoms with the same number of protons but with different number
of neutrons) which means that atoms of an element have different mass
STEP 4:
Structure of an Atom (60 minutes)
|
Activity: Small Group Discussion ( 30
minutes)
DIVIDE
students into small manageable groups
ASK students to discuss on the
following questions What
is the composition of an atom?
What
is the location of the subatomic particles?
ALLOW students
to discuss for 15 minutes
ALLOW few
groups to present and the rest to add points
not mentioned
CLARIFY and SUMMARIZE
by using the contents below
|
Composition of an Atom
Atoms
is composed of three subatomic particles:
o
Protons
§ Heavier,
larger than electrons and are positively charged o Electrons
§ Smallest,
lightest and negatively charged o Neutrons
§ Large
and massive as protons, but neutral
Table 2.1: Mass and Charge of Subatomic Particles
|
Particle
|
Mass (g)
|
Charge
|
|
Coulomb
|
Charge Unit
|
|
Electron
|
9.10938 x 10-28
|
-1.6022 x 10-19
|
-1
|
|
Proton
|
1.67262 x 10-24
|
+1.6022 x 10-19
|
+1
|
|
Neutron
|
1.67262 x 10-24
|
0
|
0
|
o
All atoms can be identified by the number of
protons and neutrons they contain o In
a neutral atom the number of protons is equal to the number of electrons, so
the atomic number also indicates the number of electrons present in the
atom
Location of Subatomic Particles According
to Different Models
The
plum pudding model of the atom (Joseph John (J.J.) Thomson) o Electrons
and protons are uniformly mixed throughout the atom
§ Atom
consist of electrons scattered in a sphere of positive charge (protons)
o
According to Thomson atoms are composed of
electrons distributed in a cloud of
positively charged material (protons)
o
The electrons are free to rotate in orbits in
the cloud, and their negative charges exactly offset the positively charged
cloud
Figure 2.1: The Plum Pudding Model
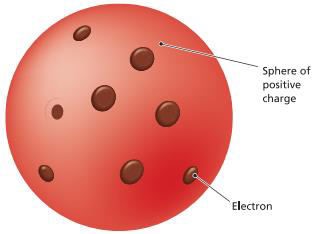
Source: Essential chemistry: Atoms, molecules and
compounds (2008).
Planetary model of the atom (Rutherford) o The
atom are composed of a tiny positively charged nucleus with even tinier
negatively charged electrons circling it
§ The protons are located in the nucleus and the electrons orbit
around the nucleus like planets around the sun
o
Electrons orbit the positively charged nucleus,
much as the planets orbit the sun
Figure 2.2: Rutherfords Model of the Atom
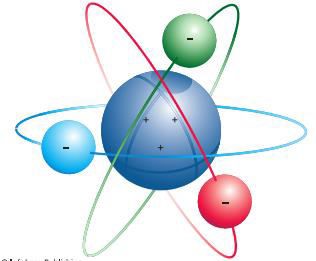
Source: Essential chemistry: Atoms, molecules and
compounds (2008).
Bohr's solar system model of the atom (Niels
Bohr) o Electrons
assume only certain orbits around the nucleus and orbits are stable o Each orbit has an energy associated with
it
o
If the orbit closest to the nucleus has an
energy E1, the next closest E2 and so on o Light is emitted when an electron jumps
from a higher orbit to a lower orbit and absorbed when it jumps from a lower to
higher orbit
Figure 2.3: Bohr's Model of the Atom
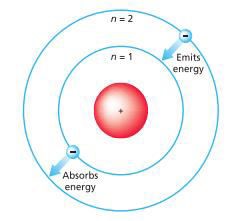
Source: Essential chemistry: Atoms, molecules and
compounds (2008).
o
The energy and frequency of light emitted or
absorbed is given by the difference between the two orbit
o
The energy of light is proportional to its
frequency and inversely proportional to its wavelength and the proportionality
constant is known as Planck's constant h
Figure 2.4: Bohr's Model of Sodium Atom having 11
Electrons
Source: Essential chemistry: Atoms, molecules and
compounds (2008).
Quantum mechanical description of an atom
o
Electrons in a certain orbital and not orbit
o
An atomic orbital has a characteristic energy,
as well as a characteristic distribution of electron density
§ Electron
density gives the probability that an electron will be found in a particular
region of an atom
o
The wave-particle nature of matter (De Broglies)
§ An
electron in an atom could be anywhere, although some locations are more likely
than others
§ Electrons
are both wave and particle o Uncertainty principle (Werner
Heisenberg)
§ It
is impossible to measure precisely both the position and the momentum of a
particle
Figure 2.5: Proposed Structure of an Atom Basing
on Newer (Quantum Model) Where the Electrons
are in a Cloud, no Specific Location.
Source: Chemistry for Pharmacy Students (2007)
Identification of Atom
Atomic
Number (Z) o The
number of protons in the nucleus of each atom of an element o The
chemical identity of an atom can be determined by its atomic number o For
example, the atomic number of nitrogen is 7 thus neutral nitrogen atom has 7
protons and 7 electrons
The mass number (A) o The total number of neutrons and protons
present in the nucleus of an atom of an element
§ Except for the most common form of hydrogen, which has one
proton and no neutrons, all atomic nuclei contain both protons and neutrons
o
The mass number = number of protons + number of
neutrons
= atomic number + number of
neutrons o The
number of neutrons in an atom is equal to the difference between the mass
number and the atomic number, or (A - Z)
§ For
example, if the mass number of a particular boron atom is 12 and the atomic
number is 5 (indicating 5 protons in the nucleus)
§ The
number of neutrons is 12 - 5 = 7 o In
most cases atoms of a given element do not all have the same mass.
o
Atoms that have the same atomic number but
different mass numbers are called isotopes
§
Hydrogen has three isotopes; hydrogen/protium
(having one proton and no neutrons), Deuterium (having one proton and one
neutron) and Tritium (having one proton and two neutrons)
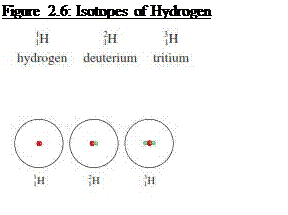
Source: Chang general chemistry: The essential
concepts. (2011).
§ Uranium
has two common isotopes with mass numbers of 235 and 238.
o
The accepted way to denote the atomic number (Z)
and mass number (A) of an atom of element X is as follows:
A x
Z
§ Example,
the isotopes of uranium
235 238
92 U U
92
o
With the exception of hydrogen, isotopes of
elements are identified by their mass numbers
§ The
two isotopes of uranium are called uranium-235 (pronounced uranium two
thirty-five) and uranium-238 (pronounced uranium two thirty-eight)
o
The chemical properties of an element are
determined primarily by the protons and electrons in its atoms;
§ Neutrons
do not take part in chemical changes under normal conditions
§ Isotopes
of the same element have similar chemistry, forming the same types of compounds
and displaying similar reactivity
STEP 5: Key
Points (10 minutes)
Atom
o
Refers to the smallest particle of an element
that can take part in a chemical reaction
According
to Daltons Atomic theory o All
matter is composed of tiny, indivisible particles called atoms
o
Atoms cannot be made or destroyed
o
All atoms of one element are identical in weight
and atoms of different elements have different weight
o
Compounds are formed by joining the atoms of two
or more elements
§ When
forming a compound, the atoms of elements combine in whole-number ratios, such
as 1 to 1, 2 to 1, 3 to 2, and so on
o
A chemical reaction is the rearrangement of
atoms
According
to modern concepts o Matter is composed of atoms which are
divisible o All
atoms of one element may not be identical in weight
An
atom is composed of three elementary particles: proton, electron, and
neutron.
The
proton has a positive charge, the electron has a negative charge, and the
neutron has no charge
Electrons
are smallest and lightest
Protons
are heavier and larger than electrons
Neutrons
are as large and massive as protons
Protons
and neutrons are located in a small region at the center of the atom, called
the nucleus
Atomic
Number (Z) is the number of protons in the nucleus of each atom of an element
The
mass number (A) is the total number of neutrons and protons present in the
nucleus of an atom of an element
STEP 6:
Evaluation (10 minutes)
What
is an Atom?
What
is the Daltons Atomic Theory?
What
is the Composition of an Atom?
Where
do the Protons, Neutrons and Electrons found in an Atom?
What
is the Difference between Atomic Number and Mass Number?
STEP 7:
Assignment (10 minutes)
|
Activity: Take Home Assignment (10 minutes)
DIVIDE
students in manageable groups
ASK the
students to work on the following assignment
Given
the following elements;
23
238
X and Y
11
92
o
What is the Atomic Number of each Element? o What
is the Mass Number of each Element?
o
What are the Number of Protons, Neutrons and
Electrons in these Atoms?
ALLOCATE time
for students to do the assignment and submit
REFER students to recommended references
|
References
Chang,
R.,& Overby, J. (2011). General
chemistry: The Essential Concepts (6th ed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rd ed.). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S., & Lutfun, N. (2007). Chemistry for pharmacy students: General,
Organic and Natural Product Chemistry. England: John Wiley & Sons Ltd.
Session 3: Electronic Configuration of an
Atom
Total Session Time: 120 minutes + 2 hours of Assignment and
Tutorial
Prerequisites
None
Learning Tasks
By the end of this session
students are expected to be able to:
Define
the term electronic configuration
Describe
quantum numbers
Describe
atomic orbitals
Explain
rules used in filling electrons in orbital
Write the electronic configuration of
different elements
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
Handout
3.1: Writing electronic configuration of elements
Handout
3.2: Electronic configuration of elements
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Definition of Electronic
Configuration
|
|
3
|
25 minutes
|
Presentation
|
Quantum Numbers
|
|
4
|
10 minutes
|
Presentation
|
Atomic Orbitals
|
|
5
|
10 minutes
|
Presentation
|
Rules of Filling Electrons
in Orbital
|
|
6
|
40 minutes
|
Presentation
Brainstorming
|
Electronic Configuration of Different
Elements
|
|
7
|
10 minutes
|
Presentation
|
Key Points
|
|
8
|
05 minutes
|
Presentation
|
Evaluation
|
|
9
|
10 minutes
|
Presentation
|
Assignment
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students to read the learning tasks and clarify
ASK students if they have any questions before continuing.
STEP 2:
Definition of Electronic Configuration (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
is electronic configuration?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Electronic
configuration o Refers
to a detailed way of showing the order in which electrons fill in around the
nucleus
§ It
describes how the electrons are distributed among the various atomic orbitals
STEP 3:
Quantum Numbers (25 minutes)
Definition and Origin of Quantum Numbers o Quantum
numbers are numbers used to describe atomic orbitals and to label electrons
that reside in them
These
numbers are derived from the mathematical solution of the Schrφdinger equation
for the hydrogen atom
Types of Quantum Numbers
There
are four types of quantum numbers o Principal quantum number, the angular
momentum quantum number, the magnetic quantum number and the spin quantum
number
Principal
quantum number, the angular momentum quantum number, and the magnetic quantum
number describe the distribution of electrons in atoms
The
spin quantum number describes the behavior of a specific electron and completes
the description of electrons in atoms
The
Principal Quantum Number (n) o Have
integral values 1, 2, 3, and so forth o The value of n determines the energy of
an orbital.
o
The principal quantum number also relates to the
average distance of the electron from the nucleus in a particular orbital
o
The larger n is, the greater the average
distance of an electron in the orbital from the nucleus and therefore the
larger the orbital
The
Angular Momentum Quantum Number (Azimuthal quantum number (ℓ)) o This
quantum number defines the shape of the orbital
o
The values of ℓ depend on the value of the
principal quantum number, n o Has
integral values of ℓ = 0 to ℓ = n - 1 for each value of n.
o
If n = 1, there is only one possible value of
ℓ that is, = n - 1 = 1 1 = 0 o If n = 2, there are two values of, given
by 0 and 1 o If
n = 3, there are three values of, given by 0, 1, and 2
o
The value of orbitals is generally designated by
the letters s, p, d, . . . c see table 3.1 below:
Table 3.1: The designation of orbitals basing on
angular momentum quantum numbers
|
ℓ
|
0
|
1
|
2
|
3
|
4
|
5
|
|
Name of orbital
|
S
|
P
|
D
|
F
|
G
|
H
|
o
Thus, if ℓ = 0, the orbitals is s; if
ℓ = 1, the orbital is p; and so on
o A
collection of orbitals with the same value of n is frequently called a
shell o One or more orbitals with the same n and
ℓ values are referred to as a sub shell o For example, the shell with n = 2 is
composed of two sub shells, ℓ = 0 and 1 (the allowed values for n =
2)
o
These sub shells are called the 2s and 2p sub
shells where 2 denotes the value of n, and s and p denote the values of ℓ
Magnetic
quantum number (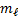 )
)
o
Describes the orientation of the orbital in
space o Has
integral values of m = - ℓ to + ℓ including 0
o
For example, if n = 3 and ℓ = 2 then the
possible values of m are -2, -1, 0, +1, +2 o The number of 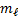 values indicates the number of orbitals in a
sub shell with a particular value
values indicates the number of orbitals in a
sub shell with a particular value
o
Example if n = 2 and ℓ = 1. The values of
n and ℓ indicate that we have a 2p sub shell, and in this sub shell we
have three 2p orbitals (because there are three values of 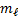 given by -1, 0, and 1)
given by -1, 0, and 1)
Spin
quantum number (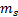 )
o The
spin quantum number has only two possible values of
)
o The
spin quantum number has only two possible values of 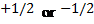
Figure 3.2: The (a) clockwise and (b)
counterclockwise spins of an electron: The upward and downward arrows are used
to denote the direction of spin
Source: Chang
general chemistry: The Essential Concepts (2011)
STEP 4:
Atomic Orbitals (10 minutes)
Atomic
Orbitals o Refers
to a region where there is high probability of locating an electron
§ Region
with massive distribution of the electron density
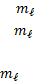 Relationship
between the atomic number and the quantum numbers; o When
ℓ = 0, (2ℓ + 1) = 1 and there is only one value of thus, we have an s orbital o When ℓ = 1, (2ℓ + 1) 5 = 3,
so there are three values of or three p orbitals, labeled px, py, and
pz
Relationship
between the atomic number and the quantum numbers; o When
ℓ = 0, (2ℓ + 1) = 1 and there is only one value of thus, we have an s orbital o When ℓ = 1, (2ℓ + 1) 5 = 3,
so there are three values of or three p orbitals, labeled px, py, and
pz
o
When ℓ = 2, (2ℓ + 1) = 5 and there
are five values of and the corresponding
five d orbitals are labeled with more elaborate subscripts
Table 3.2: The Relation between Quantum Numbers
and Atomic Orbitals
|
N
|
ℓ
|
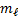
|
Number of orbitals
|
Atomic orbital
designation
|
|
1
|
0
|
0
|
1
|
1s
|
|
2
|
0
|
0
|
1
|
2s
|
|
|
1
|
-1,0,1
|
3
|
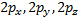
|
|
3
|
0
|
0
|
1
|
3s
|
|
|
1
|
-1,0,1
|
3
|
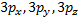
|
|
|
2
|
-2,-1,0,1,2
|
5
|
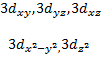
|
s
Orbitals o Spherical in shape but differ in size,
which increases as the principal quantum number increases
Figure 3.3: s orbital
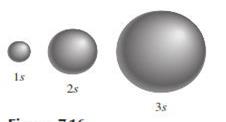
Source: Chang
general chemistry: The Essential Concepts (2011)
p
Orbitals o These
orbitals are identical in shape and energy, but their orientations are
different o The p orbitals of higher principal
quantum numbers have a similar shape
Figure 3.4: p orbital
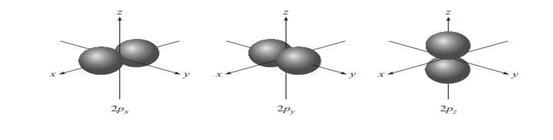
Source: Chang
general chemistry: The Essential Concepts (2011).
d
Orbitals and Other Higher-Energy Orbitals
Figure 3.5: d orbital
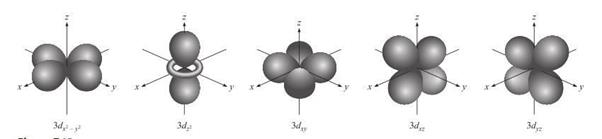
Source: Chang
general chemistry: The Essential Concepts (2011).
STEP 5:
Rules of Filling Electrons in Orbitals (10 minutes)
Several
rules govern the filling of electrons in orbitals o Aufbaus Principle
§ Electrons
occupy orbitals with lowest energy first
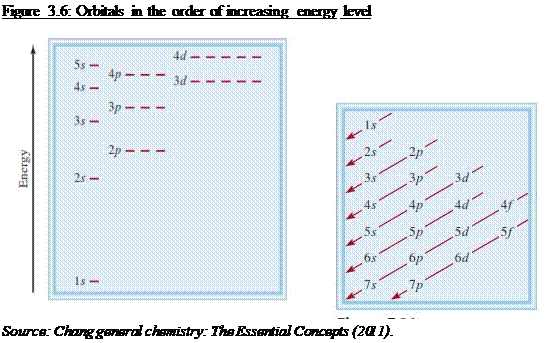
o Pauli Exclusion Principle
§ No
two electrons in an atom can have the same four quantum numbers
§ If
2 electrons occupy the same energy level they must have opposite spins
Figure 3.7: The (a) parallel and (b) antiparallel
spins of two electrons. In (a), the two magnetic
fields reinforce each other. In (b), the two magnetic fields cancel each other.
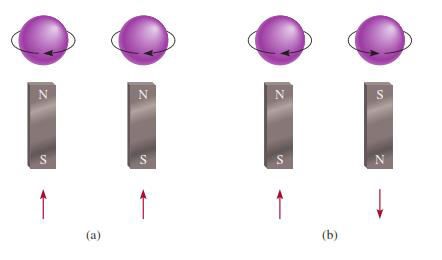
Source: Chang general chemistry: The Essential
Concepts (2011). o Hunds
Rule
§ Electrons
that occupy orbitals of the same energy will have the maximum number of
electrons with the same spin before pairing is done
STEP 6:
Electronic Configuration of Different Elements (40 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are the electronic configurations of hydrogen and helium?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
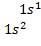 For
hydrogen with 1 electron its electronic configuration is For
helium with 2 electron its electronic configuration is
For
hydrogen with 1 electron its electronic configuration is For
helium with 2 electron its electronic configuration is
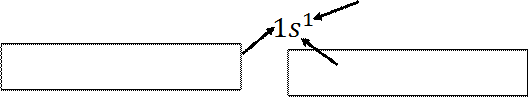 denotes the principal quantum denotes the angular momentum
denotes the principal quantum denotes the angular momentum
number n quantum
number ℓ
|
denotes
the number of electrons in the orbital or sub shell
|
The electron configuration of hydrogen and
helium can also be represented by an orbital diagram that shows the spin of the
electron;
H

 He and not or
He and not or 
(a) (b) (c)
For helium atom; the configuration in (a) is
physically acceptable but the diagrams (b) and (c) are ruled out by the Pauli
Exclusion Principle
o
In (b), both electrons have the same upward spin
and would have the quantum numbers (1, 0, 0, 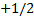 )
)
o
In (c), both electrons have downward spins and
would have the quantum numbers (1,
0, 0,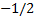 )
)
o
Configuration (a) is physically acceptable,
because one electron has the quantum numbers (1, 0, 0,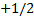 )
and the other has (1, 0, 0,
)
and the other has (1, 0, 0,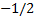 )
)
If the two electrons in the orbital have the
same, or parallel spins their net magnetic fields reinforce each other
o
Paramagnetic substances are those that contain
net unpaired spins and are attracted by a magnet
If the electron spins are paired, or ant
parallel to each other the magnetic effects cancel out
o
Diamagnetic substances do not contain net
unpaired spins and are slightly repelled by a magnet
 Refer
students to Handout 3.1: Writing
Electronic Configuration of Elements for further reading
Refer
students to Handout 3.1: Writing
Electronic Configuration of Elements for further reading
Uses of noble gas core in writing the
electronic configuration
The electronic configurations of all elements
except hydrogen and helium are represented by a noble gas core, which shows in
brackets
o
the noble gas element that most nearly precedes
the element being considered, followed by the symbol for the highest filled sub
shells in the outermost shells
The 4s sub shell is filled before the 3d sub
shell in a many-electron atom (see Figure 3.6)
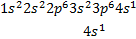 The
electron configuration of potassium (Z = 19) is
The
electron configuration of potassium (Z = 19) is
The electron configuration of potassium can be
also written [Ar] where [Ar]
denotes the argon core
o
Because 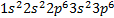 is the electron configuration of argon
is the electron configuration of argon
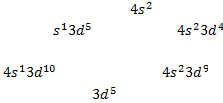 The
electron configuration of calcium (Z = 20) can be written as [Ar]
The
electron configuration of calcium (Z = 20) can be written as [Ar]
The electron configuration of chromium (Z = 24)
is [Ar]4 and not [Ar] , as expected
Also
for copper the electron configuration is [Ar]
rather than [Ar] o A slightly greater stability is
associated with the half-filled ( )
and completely filled (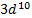 )
sub shells
)
sub shells
§
The electrons are more strongly
attracted by the nucleus when they have the 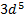 configuration
configuration
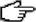 Refer
students to Handout 3.2: Electronic
configuration of elements for further reading
Refer
students to Handout 3.2: Electronic
configuration of elements for further reading
STEP 7: Key
Points (5 minutes)
o
Electronic configuration is a detailed way of
showing the order in which electrons fill in around the nucleus
o
Atomic Orbitals is a region where there is high probability of
locating the electron o Quantum numbers are used to describe
atomic orbitals and to label electrons that reside in them
o
Principal quantum number, the angular momentum
quantum number and the magnetic quantum number describe the distribution of
electrons in atoms
o
The spin quantum number describes the behavior
of a specific electron and completes the description of electrons in atoms
Several rules govern the filling of electrons in
orbitals o Aufbau
principle o Pauli
exclusion principle o Hunds rule
STEP 8:
Evaluation (5 minutes)
What is electronic configuration?
What are quantum numbers?
What are atomic orbitals?
What are the rules used in filling electrons in
orbital?
STEP 9:
Assignment (10 minutes)

References
Chang,
R.,& Overby, J. (2011). General
chemistry: The Essential Concepts (6th ed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rd ed). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S, & Lutfun, N. (2007). Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
 Handout 3.1: Writing Electronic Configuration of Elements
Handout 3.1: Writing Electronic Configuration of Elements
|
Element
|
Electronic diagram
|
|
Beryllium z = 4
|
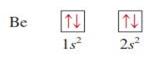
|
|
Boron z = 5
|
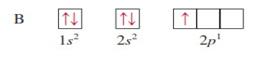
|
|
carbon z = 6
|
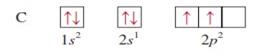
|
|
Nitrogen z = 7
|
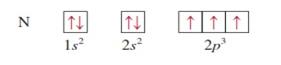
|
|
Oxygen z = 8
|
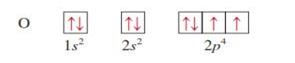
|
|
Fluorine z = 9
|

|
|
Neon z = 10
|

|
|
Chromium z = 24
|

|
|
Copper z = 29
|

|
Session 4: Arrangement and General Trends of
elements in the Periodic Table
Total Session Time: 120 minutes+ 2 hours of Assignment and Tutorial
Prerequisites
None
Learning Tasks
By the end of this session
students are expected to be able to:
Define
the term periodic table
Describe
the arrangement of elements in periodic
table Describe
the trend of the periodic table
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
Handout
4.1 Periodic table
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Definition of Periodic Table
|
|
3
|
05 minutes
|
Presentation
|
Arrangement of Elements in the
Periodic Table
|
|
4
|
60 minutes
|
Presentation Small group discussion
|
Physical Trends of the
Periodic Table
|
|
5
|
20 minutes
|
Presentation
|
Chemical Trends of the
Periodic Table
|
|
6
|
05 minutes
|
Presentation
|
Key Points
|
|
7
|
10 minutes
|
Presentation
|
Evaluation
|
|
8
|
10 minutes
|
Presentation
|
Assignment
|
SESSION CONTENTS
STEP1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students to read the learning tasks and clarify
ASK students if they have any questions before continuing
STEP 2:
Definition of the Term Periodic Table (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
is the periodic table?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Periodic
table
Refers
to a table in which elements are arranged by the order of increasing atomic
number
STEP 3:
Arrangement of Elements in the Periodic Table (5minutes)
Periodic law o States that the properties of the
elements are a periodic function of their atomic weights
o
Developed by Dmitri Ivanovitch Mendelιev
Atoms
are arranged in the order of increasing atomic number o Atomic
number
§ Refers
to the number of protons in the nucleus of an atom
The
vertical columns of the table are called groups or families o Numbered
from 1 to 18
o Elements
in the same group have the same number of outer shell electrons, and hence
similar chemical properties
The
horizontal rows of the table are called periods o Numbered from 1 to 7
o Elements in the same period have
electrons in the same outer shell
Refer students to
Handout 4.1: Periodic table for further reading
STEP 4:
Physical Trends of the Periodic Table (60 minutes)
|
Activity: Small Group Discussion ( 30
minutes)
DIVIDE
students into small manageable groups
ASK students
to discuss on the following question
What
are the trends of elements in the periodic table?
ALLOW students
to discuss for 15 minutes
ALLOW few
groups to present and the rest to add points
not mentioned
CLARIFY and SUMMARIZE
by using the contents below
|
Core
Charge
o
Refers to the attraction that an outer shell
electron feels towards the nucleus o Down the group it is constant
§ There
is always equal number of electron in the outer shell example in the alkaline
metals there is always one electron in the outer shell.
o
Across a period it increases
§ There
are more electrons in the outer shell which are greatly attracted to the
nucleus
Atomic
radius or size o Refers to the diameter of an atom
§
One-half the distance between the two nuclei in
two adjacent metal atoms
Figure 4.1: The atomic radius
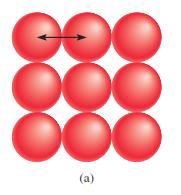
Source: Chang general chemistry: The Essential
Concepts (2011).
§
For elements that exist as simple diatomic
molecules, the atomic radius is one-half the distance between the nuclei of the
two atoms in a particular molecule.
Figure 4.2: The atomic radius of diatomic
molecules
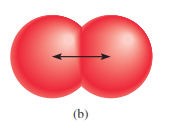
Source: Chang general chemistry: The Essential
Concepts (2011).
o
Down the group it increases
§ There
is an increase in the number of shells o Across
the period it decreases
§ There
is an increase in core charge, the outer shell electrons are attracted closer
to the nucleus (its the same shell but there are more electrons in the shell
as you move across the period)
Figure 4.3: The atomic radius of diatomic
molecules
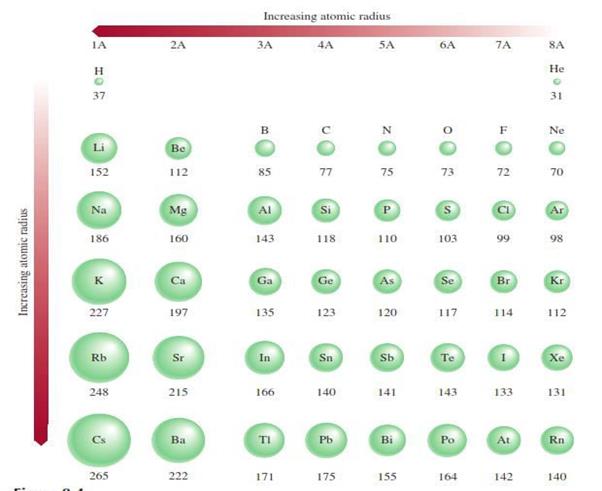
Source: Chang general chemistry: The Essential
Concepts (2011)
Ionisation Energy o Refers to the minimum amount of energy
required to remove the highest energy electron from an atom (energy to steal an
electron) o The
minimum energy (in kJ/mol) required to remove an electron from a gaseous atom

 first
ionization energy second ionization energy
third ionization energy § The size of the
atom is increasing, the attraction is weaker between the outer shell electrons
and the nucleus therefore electrons are easier to remove
first
ionization energy second ionization energy
third ionization energy § The size of the
atom is increasing, the attraction is weaker between the outer shell electrons
and the nucleus therefore electrons are easier to remove
o
Across a period it increases
§ There
is an increase in core charge, the attraction is greater between the outer
shell electrons and the nucleus. Therefore electrons are harder to remove
Figure 4.4: The atomic radius of diatomic
molecules
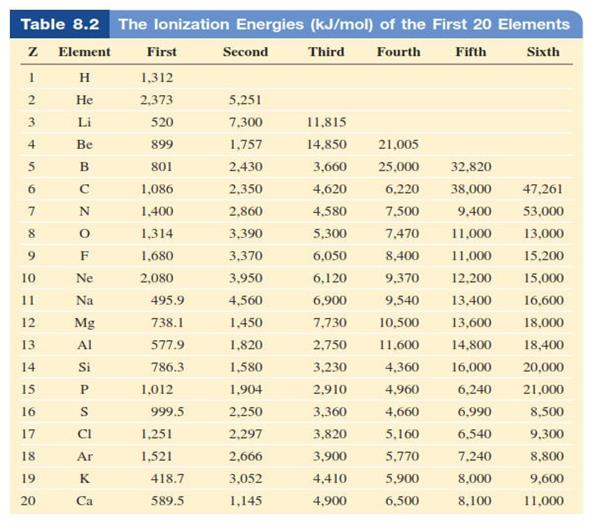
Source: Chang general
chemistry: The Essential Concepts (2011).
Electro
negativity o Refers
to measure of the ability of an atom to attract an electron towards itself.
(love of electrons)
o
Down the group it decreases
§ The
electrons are further from the nucleus, there is a weaker attraction o Across
a period it increases
§ There
is an increase in core charge, there is a greater attraction of the outer shell
electrons to the nucleus
STEP 5:
Chemical Trends of the Periodic Table (20 minutes)
Elements
in the same group exhibits many, but not all the chemical properties behaviour
because they have similar valence electron configurations
o
Lithium, for example, exhibits many, but not
all, of the properties characteristic of the alkali metals
o
Beryllium is a somewhat atypical member of Group
2A
o
The difference can be attributed to the
unusually small size of the first element in each group
o
There is small variation in chemical properties
for elements in Groups 1A and 2A, which are all metals, and to the elements in
Groups 7A and 8A, which are all nonmetals
o
There is a greater variation for the elements in
Groups 3A through 6A, where the elements change either from non-metals to
metals or from non-metals to metalloids
The diagonal relationship
Refers
to similarities between pairs of elements in different groups and periods of
the periodic table
Specifically,
the first three members of the second period (Li, Be, and B) exhibit many
similarities to those elements located diagonally below them in the periodic
table (Figure
4.4)
Figure 4.5: The diagonal relationship
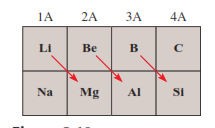
Source: Chang general chemistry: The Essential
Concepts (2011)
The
reason for this is the closeness of the charge densities of their cations o Charge
density is the charge of an ion divided by its volume
Cations
with comparable charge densities react similarly with anions and therefore form
the same type of compounds
o
The chemistry of lithium resembles that of
magnesium in some ways; the same holds for beryllium and aluminium and for
boron and silicon
Properties of Oxides
As
the metallic character of the elements decreases from left to right across the
period, their oxides change from basic to amphoteric to acidic
Metallic oxides are usually basic o Sodium
oxide reacts with water to form hydroxide
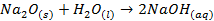
o
Magnesium oxide is quite insoluble; it does not
react with water to any appreciable extent
§
It reacts with acids in a manner that resembles
an acid-base reaction
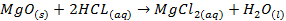
o
Aluminium oxide is even less soluble than
magnesium oxide; it does not react with water too
Most
oxides of non-metals are acidic o They react with water to form phosphoric
acid (H3PO4), sulphuric acid (H2SO4),
and perchloric acid (HClO4)
The
intermediate properties of the oxides (as shown by the amphoteric oxides) are
exhibited by elements whose positions are intermediate within the period.
o
Aluminium oxide (Al2O3) is
classified as an amphoteric oxide because it has properties of both acids and
bases
§ It
shows basic properties by reacting with acids
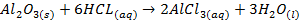
§
It also exhibits acidic properties by reacting
with bases, but the neutralization reaction produces salt only
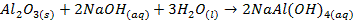
o
Other amphoteric oxides are ZnO, BeO, and Bi2O3
Silicon dioxide is insoluble and does not react
with water o It
has acidic properties, however, because it reacts with very concentrated bases
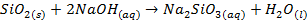
Certain
oxides such as CO and NO are neutral; that is, they do not react with water to
produce an acidic or basic solution
As
the metallic character of the elements increases from top to bottom within a
group of representative elements then the basic property of oxides increases
STEP 6: Key
Points (5 minutes)
Periodic table
o Refers
to a table in which elements are arranged by the order of increasing atomic
number
Atoms are arranged in the order of increasing
atomic number in a table o The vertical columns of the table are
called groups or families o The horizontal rows of the table are
called periods
There are four categories of oxides in the
periodic table
o
Basic oxides (example 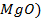 ,
amphoteric oxides (example
,
amphoteric oxides (example 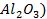 ,
neutral oxides
,
neutral oxides
(example 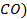 and Acidic oxides (example
and Acidic oxides (example 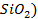
As
the metallic character of the elements decreases from left to right across the
period, their oxides change from basic to amphoteric to neutral to acidic
As
the metallic character of the elements increases from top to bottom within a
group of representative elements then the basic property of oxides increases
STEP 7:
Evaluation (10 minutes)
What
is periodic table?
What
is the periodic law?
What
are the trends of elements in a periodic table?
STEP 8:
Assignment (10 minutes)

References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6thed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rded). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S.,& Lutfun, N. (2007).Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
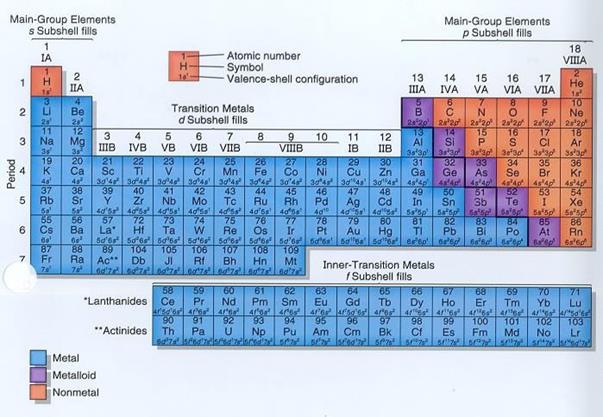
Session 5: Characteristics and Uses of Group One
Elements in Pharmacy
Total Session Time: 120 minutes
Prerequisites
None
Learning Tasks
By the end of this session
students are expected to be able to:
List
group one elements
Describe
physical characteristics of group one elements
Describe
chemical characteristics of group one elements Describe
the importance of Group one elements in pharmacy
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Group One Elements
|
|
3
|
20 minutes
|
Presentation
Buzzing
|
Physical Characteristics of Group One
Elements
|
|
4
|
60 minutes
|
Presentation Small Group
|
Chemical Characteristics of
Group One
|
|
|
|
Discussion
|
Clements
|
|
5
|
15 minutes
|
Presentation
|
The Use of Group One
Elements in Pharmacy
|
|
6
|
10 minutes
|
Presentation
|
Key Points
|
|
7
|
05 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students to read the learning tasks and clarify
ASK students if they have any questions before continuing
STEP 2:
Group One Elements (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are the group one elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
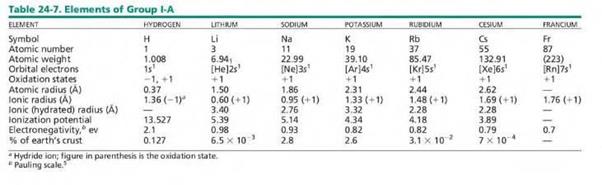
These are Alkali Metals (Group 1A) o Refers
to Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Caesium (Cs) and
Francium (Fr)
o Hydrogen
may also be placed in this group as it shares some features
STEP 3:
Physical Characteristics of Group One Elements (20 minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
are the physical characteristics of group one elements?
ALLOW few
pairs to respond and let other pairs to add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
They
are found in group 1 of the periodic table
Their
outermost electrons fill the s orbital
Their atoms have a single electron in their
outermost level (1 valence electron)
References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6th ed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rd ed.). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S, & Lutfun, N. (2007). Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21st ed.). Baltimore, MA. A Wolters
Kluwer Company.
Session 6: Characteristics and Uses of Group Two
Elements in Pharmacy
Total Session Time: 120 minutes
Prerequisites
None
Learning Tasks
By the end of this session students are expected to be able
to:
List
the group two elements
Describe
the physical characteristics of group two elements
Describe
the chemical characteristics of group two elements
Describe
the importance of Group two elements
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Group Two Elements
|
|
3
|
20minutes
|
Presentation
Buzzing
|
Physical Characteristics of Group Two
Elements
|
|
4
|
60minutes
|
Presentation Small Group
|
Chemical Characteristics of
Group Two
|
|
|
|
Discussion
|
Elements
|
|
5
|
15 minutes
|
Presentation
|
The Use of Group Two Elements in Pharmacy
|
|
6
|
05 minutes
|
Presentation
|
Key Points
|
|
7
|
10 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing
STEP 2:
Group Two Elements (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are group two elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Alkaline Earth Metals (Group 2A) o Refers
to beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), Barium (Ba),
and radium (Ra)
STEP 3:
Physical Characteristics of Group Two Elements (20minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
are the physical characteristics of group two elements?
ALLOW few
pairs to respond and let other pairs add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
They
are never found un-combined in nature
They
have two valence electrons
Melt at extremely high temperatures
Group
2 elements have higher ionization energies than Group 1 elements
Ionization
energy decrease down the group

STEP 4:
Chemical Characteristics of Group Two Elements (60 minutes)
|
Activity: Small Group Discussion ( 40
minutes)
DIVIDE
students into small manageable groups
ASK students
to discuss on the following question
What
are the chemical characteristics of group two elements?
ALLOW students
to discuss for 20 minutes
ALLOW few
groups to present and the rest to add points
not mentioned
CLARIFY and SUMMARIZE
by using the contents below
|
Chemical characteristics of group two elements include:
Most
of the elements reduce H2 to form ionic hydrides
Somewhat
less reactive than the alkali metals
Due
to their higher effective nuclear charge and smaller size Group 2 elements are
strong reducing agents
The alkali earth metals reduce O2 to
form oxides o The reactivity of the alkaline earth
metals toward oxygen increases from Be to Ba
§ Beryllium
and magnesium form oxides (BeO and MgO) only at elevated temperatures
§ CaO,
SrO, and BaO form at room temperature
Magnesium reacts with acids in aqueous solution,
liberating hydrogen gas
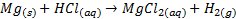
The reactivity of alkaline earth metals with
water vary quite markedly o The
larger metals reduce water to form H2 gas o Beryllium
does not react with water o Magnesium reacts slowly with steam
o Calcium,
strontium, and barium are reactive enough to attack cold water

The
oxides of Group 2 elements form basic solutions
Strontium-90
is a radioactive isotope
STEP 5: The
Use of Group Two Elements in Pharmacy (15 minutes)
Physiological role o Magnesium
ions and calcium ions are important for transportation of material in and
outside of the cell
o
Magnesium and calcium are also very crucial for
the normal function of the heart
Preparation pharmaceuticals o Magnesium
insoluble compounds are used in the preparation of gastric antacids o Calcium
is also important for preparation of antacids o Calcium is important for preparation of
antacids and calcium replenishers o Magnesium hydroxide has been used as an
ingredient in cathartic preparation o Magnesium sulfate as an
anticonvulsant
Medical
analysis and diagnosis o Barium hydroxide is applied in analytical
and synthetic operations
STEP 6: Key Points (5 minutes) Alkaline
Earth Metals (Group 2A) o Refers to beryllium (Be), magnesium
(Mg), calcium (Ca), strontium (Sr), Barium (Ba), and radium (Ra)
They
are never found un-combined in nature
They
have two valence electrons
Melt
at extremely high temperatures
The
metals reduce O2 to form oxides
The
oxides of Group 2 elements form basic solutions
Alkaline
earth metals and their compounds have Physiological role and have been used in
preparation of pharmaceuticals and have a role in Medical analysis and
diagnosis
STEP 7:
Evaluation (10 minutes)
What
are the group two elements?
What
are the physical characteristics of group two elements?
What
are the chemical characteristics of group two elements?
What
is the importance of group two elements?
References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6thed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rded.). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S., & Lutfun, N. (2007).Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21sted.). Baltimore, MA. A Wolters Kluwer
Company.
Session 7: Characteristics and Uses of Transition Elements in Pharmacy
Total Session Time: 120 minutes
Prerequisites
None
Learning Tasks
By the end of this session students are expected to be able
to:
List
the transition elements
Describe
the physical characteristics of transition elements
Describe
the chemical characteristics of transition elements Describe uses of transition elements
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
Handout
7.1: Properties of transition elements
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
tasks
|
|
2
|
15 minutes
|
Presentation
Brainstorming
|
Transition Elements
|
|
3
|
40 minutes
|
Presentation
Small group
|
Physical Characteristics of
Transition
|
|
|
|
discussion
|
Elements
|
|
4
|
20 minutes
|
Presentation
Brainstorming
|
Chemical Characteristics of Transition
Elements
|
|
5
|
20 minutes
|
Presentation
|
Uses of Transition Elements
in Pharmacy
|
|
6
|
10 minutes
|
Presentation
|
Key Points
|
|
7
|
10 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing
STEP 2:
Transition Elements (15 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are Transition elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Transition
Elements o Refers to elements in the B
families
Group
IB transition elements o Copper (Cu), Silver (Ag) and Gold (Au)
Group
IIB transition elements o Zinc (Zn), Cadmium (cd) and Mercury (Hg)
Group
IIIB transition elements o Scandium
(Sc), Yttrium (Y) and Lanthanum (La)
Group
IVB transition elements o Titanium
(Ti), Zirconium (Zr) and Hafnium (Hf)
Group
VB transition elements o Vanadium
(V), Niobium (Nb) and Tantalum (Ta)
Group
VIB transition elements o Chromium
(Cr), Molybdenum (Mo) and Tungsten (W)
Group
VIIB transition elements o Manganese
(Mn), Technetium (Tc) and Rhenium (Re)
Group
VIIIB transition elements o Iron (Fe), Cobalt (Co) and Nickel (Ni)
STEP 3:
Physical Characteristics of Transition Elements (40 minutes)
|
Activity: Small Group Discussion ( 30
minutes)
DIVIDE
students into small manageable groups
ASK students
to discuss on the following question
What
are the characteristics of Transition elements?
ALLOW students
to discuss for 15 minutes
ALLOW few
groups to present and the rest to add points
not mentioned
CLARIFY and SUMMARIZE
by using the contents below
|
Characteristics of transition
elements include the following:
Most
transition elements are found combined with other elements in ores All of them are
metals
Most
transition metals have higher melting points than the representative
elements
They
are good conductors of heat and electricity
The
compounds of transition metals are usually brightly colored and are often used
to color paints
o
Iron (II) compounds are usually green in the
hydrated state and white in the anhydrous state
o
Iron (III) salts are usually yellow to brown in
the hydrated state but vary in colour when anhydrous
Have
partially filled d orbital
Have
variable oxidation states
Have
spectroscopic, magnetic or structural features resulting from partially
occupied d orbital.
They
have catalytic properties
Refer students to
Handout 7.1: Properties of transition elements for further reading
STEP 4:
Chemical Characteristics of Transition Elements (20 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are the chemical characteristics of Transition elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Chemical characteristics of transition elements include the
following:
Titanium
forms three oxides (TiO, Ti2O3, and TiO2) and
corresponding binary salts
The
soluble salts of divalent and trivalent titanium are violet or red and are
powerful reducing agents
Aqueous
solutions of iron (III) salts hydrolyze strongly to give acid solutions
Iron
(III) salts undergo slight hydrolysis and are oxidized easily in solution
Transition metals have a distinct tendency to
form complex ions o A
coordination compound typically consists of a complex ion and counter ion o Most,
but not all, of the metals in coordination compounds are transition metals
A
transition metal atom (in either its neutral or positively charged state) acts
as a Lewis acid, accepting (and sharing) pairs of electrons from the Lewis
bases
STEP 5: The
Use of Transition Elements in Pharmacy (20 minutes)
Physiological
role o Copper
is an essential trace element and small quantities enhance the physiological
utilization of iron
o
Manganese is an essential trace element, being
necessary for the activation of a variety of enzymes such as pyruvate
carboxylase
o
Iron is an essential trace element
§
It is the important element in the
transportation of oxygen by haemoglobin
§
It functions in various cytochromes, which are
essential oxidative enzymes of the body cells
Preparation
of pharmaceuticals o Various copper compounds are used as
fungicides and insecticides, and they are particularly effective algaecides o Silver
sulfadiazine is used topically as a germicide
§ Because
of the ability of silver ion to precipitate protein and chloride in the affected
tissue, silver compounds such as silver nitrate are employed to provide local
germicidal action
o
Gold compounds are employed in the treatment of
lupus erythematosus and rheumatoid arthritis
o
Zinc is used in the treatment of various
external surfaces of the body and in wound healing, taste acuity, and various
ophthalmic problems
o
Soluble cadmium compounds are astringent
o
CdSO4 has been used both as a topical
astringent and for eye infections o Potassium Permanganate USP is used as a
local anti-infective and is also an astringent, powerful deodorant and cleanser
o
Titanium dioxide is also a popular ingredient in
various lotions and creams for the prevention of sunburn
o
Iron (III) compounds are astringent
o
Sodium nitroprusside USP, 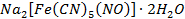 is used as a vasodilator o Ferrous
Fumarate (Tablets), Ferrous Gluconate (Tablets, Capsules, and Elixir), Ferrous
Sulphate (Oral Solution, Syrup, and Tablets) and Dried Ferrous Sulphate are
used as Hematinics
is used as a vasodilator o Ferrous
Fumarate (Tablets), Ferrous Gluconate (Tablets, Capsules, and Elixir), Ferrous
Sulphate (Oral Solution, Syrup, and Tablets) and Dried Ferrous Sulphate are
used as Hematinics
o
Iron Dextran Injection (a colloidal iron (III)
hydroxide with partially hydrolyzed dextran) and Iron Sorbitex Injection (a
complex of iron with sorbitol and citric acid) are cited in the USP as
injectable forms for patients with poor gastrointestinal tolerance or poor
absorption of iron
As
medical aids o Titanium dioxide TiO2 is used as a
solar-ray protective
o
Tantalum is unaffected by the body fluids and is
used in sheet form for the surgical repair of bones
o
Radioactive technetium, 99Tc is used
for diagnosis in various forms
As
aids in scientific studies and research o The radioactive isotopes have been
employed in mineral metabolism studies o Copper(II) sulphate is the basis for
Fehling's and Benedict's Solutions, the classic test solutions for reducing
sugars
STEP 6: Key
Points (10 minutes)
Transition
Metals o Refers
to elements in the B families
o
Include Copper (Cu), Silver (Ag), Gold (Au),
Zinc (Zn), Cadmium (cd), Mercury
(Hg), Scandium (Sc), Yttrium
(Y), Lanthanum (La), Titanium (Ti), Zirconium (Zr),
Hafnium (Hf), Vanadium (V),
Niobium (Nb), Tantalum (Ta), Chromium (Cr),
Molybdenum (Mo), Tungsten (W),
Manganese (Mn), Technetium (Tc), Rhenium
(Re), Iron (Fe), Cobalt (Co) and
Nickel (Ni)
All
of them are metals
Most transition metals have higher melting
points than the representative elements They
are good conductors of heat and electricity
The
compounds of transition metals are usually brightly colored and are often used
to color paints
Transition
metals have a distinct tendency to form complex ions
STEP 7:
Evaluation (10 minutes)
What
are the transition elements?
What
are the physical characteristics of Transition elements?
What
are the chemical characteristics of transition elements?
What
are the uses of transition elements?
References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6thed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rded.). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S &Lutfun, N. (2007).Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21sted.). Baltimore, MA. A Wolters Kluwer
Company.
Session 8: Characteristics and Uses of Group Three
Elements in Pharmacy
Total Session Time: 60 minutes
Prerequisites
None
Learning Tasks
By the end of this session students are expected to be able
to:
List
the group three elements
Describe
the physical characteristics of group three elements
Describe
the chemical characteristics of group three elements
Describe
the use of Group three elements in pharmacy
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Group Three Elements
|
|
3
|
10 minutes
|
Presentation
Buzzing
|
Physical Characteristics of Group Three
Elements
|
|
4
|
15 minutes
|
Presentation
Brainstorming
|
Chemical Characteristics of Group Three
Elements
|
|
5
|
10 minutes
|
Presentation
|
The Use of Group Three Elements in
Pharmacy
|
|
6
|
05 minutes
|
Presentation
|
Key Points
|
|
7
|
10 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Group Three Elements (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are group three elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
These
are Boron family (Group 3A or 13) o Boron (B), Aluminium (Al), Gallium (Ga),
Indium (In) and Thallium (Tl)
STEP 3:
Physical Characteristics of Group Three Elements (10 minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
are the physical characteristics of group three elements?
ALLOW few
pairs to respond and let other pairs to add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
Atoms
in this family have 3 valence electrons
Some
are metalloid (boron), and the rest are metals
Aluminum
is the most abundant metal in the earths crust
Larger
Group 3 elements exhibit multiple oxidation states
Figure 8.1: Properties of group three elements
Source: Remington: The science and practice of
pharmacy (2015)
STEP 4:
Chemical Characteristics of Group Three Elements (15 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are the chemical characteristics of group three elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
They
react sluggishly, if at all, with water
When
strongly heated in pure 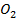 ,
all members form oxides
,
all members form oxides
All
members reduce halogens
Boron
does not form binary ionic compounds and is unreactive toward oxygen gas and
water
Aluminium
readily forms aluminium oxide when exposed to air
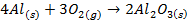
Aluminium
reacts with hydrogen to form 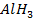
Aluminium
that has a protective coating of aluminium oxide is less reactive than
elemental aluminium
o Aluminium forms only tri-positive ions o It
reacts with hydrochloric acid to form hydrogen gas
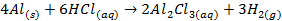
STEP 5: The
Use of Group Three Elements in Pharmacy (10 minutes)
As ingredients in various pharmaceutical
formulations o Boric acid is included in topical
preparations as an anti-infective o Elemental aluminium is employed topical
preparations as a protective o Sodium
borate is an ingredient of cold creams, eye washes, and mouthwashes o Many insoluble aluminium compounds are
used in preparing gastric antacids o Preparing
aluminium salts used for various skin conditions
§ Kaolin
is used as an adsorbent and demulcent
§ Bentonite
is useful as a suspending agent
STEP 6: Key
Points (5 minutes)
These
are Boron family (Group 3A or 13) o Refers Boron (B), Aluminium (Al),
Gallium (Ga), Indium (In) and Thallium (Tl)
Atoms
in this family have 3 valence electrons
Some
are metalloid (boron), and the rest are metals
Aluminum
is the most abundant metal in the earths crust
The
elements react sluggishly, if at all, with water
When
strongly heated in pure  ,
all members form oxides
,
all members form oxides
All
members reduce halogens
The
group three elements are used in pharmacy as ingredients in various
pharmaceutical formulations
STEP 7:
Evaluation (10 minutes)
What
are the group three elements?
What
are the physical characteristics of group three elements?
What
are the chemical characteristics of group three elements?
What
are the uses of Group three elements in pharmacy?
References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6th ed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rd ed.). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S, & Lutfun, N. (2007). Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21st ed.). Baltimore, MA. A Wolters
Kluwer Company.
Session 9: Characteristics and Uses of Group Four
Elements in Pharmacy
Total Session Time: 60 minutes
Prerequisites
None
Learning Tasks
By the end of this session students are expected to be able
to:
List
group four elements
Describe
the physical characteristics of group four elements
Describe
the chemical characteristics of group four elements
Describe
the importance of group four elements
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Group Four Elements
|
|
3
|
10 minutes
|
Presentation
Buzzing
|
Physical Characteristics of Group Four
Elements
|
|
4
|
10 minutes
|
Presentation
Brainstorming
|
Chemical Characteristics of Group Four
Elements
|
|
5
|
20 minutes
|
Presentation
|
The Use of Group Four Elements in Pharmacy
|
|
6
|
05 minutes
|
Presentation
|
Key Points
|
|
7
|
05 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Group Four Elements (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are group four elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Carbon Family (Group 4A or 14) o This
family includes carbon (C), Silicon (Si) and germanium (Ge), Tin (Sn) and lead
(Pb)
STEP 3:
Physical Characteristics of Group Four Elements (10 minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
are the physical characteristics of group four elements?
ALLOW few
pairs to respond and let other pairs add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
Atoms
of this family have 4 valence electrons
Some
are non-metal (carbon), metalloids (Silicon and germanium), and metals (Tin and
lead)
Tin and lead are the two heaviest elements
Carbon
is the basis of life; and is also found in all living things
Carbon
forms predominantly covalent bonds, but the larger members of the group form
bonds with increasing ionic character
Carbon has the ability to bond to itself, and to
form multiple bonds (catenation) o Catenation is a process whereby carbon
bonds to itself to form stable chains, branches, and rings
Figure 9.1: Properties of group four elements
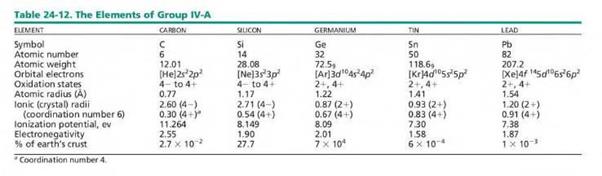
Source: Remington: The science and practice of
pharmacy (2015)
Elements
of this group also exhibit multiple oxidation states o The
Group 4A elements form compounds in both the +1 and +4 oxidation states
o
For carbon and silicon, the +4 oxidation state is
the more stable one
§  is
more stable than
is
more stable than
o
In tin compounds, the +4 oxidation state is only
slightly more stable than the +2 oxidation state
o
In lead compounds, the +2 oxidation state is the
more stable one
STEP 4:
Chemical Characteristics of Group Four Elements (10 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are the chemical characteristics of group four elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
The
Group 4A(14) elements are oxidized by halogens
The metallic elements tin and lead do not react
with water, but they do react with acids to liberate hydrogen gas

The
elements are also oxidized by O2
STEP 5: The
Use of Group Four Elements in Pharmacy (20 minutes)
Their
compounds are used in preparation of pharmaceuticals o Carbon
is used in preparation of activated charcoal
o
Sodium bicarbonate and the slightly soluble
carbonates or basic carbonates of calcium, magnesium, and aluminium are used as
gastric antacids
o
Potassium bicarbonate is used as source of
potassium ion in electrolyte replenishers. o Bismuth sub carbonate is an astringent
and protective
o
Ammonium carbonate is an effective reflex
stimulant and expectorant o Talc (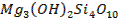 )
is the softest mineral that cleave easily to give the
)
is the softest mineral that cleave easily to give the
characteristic smooth feel
and it adheres readily to the skin, is
chemically inert, and has very low adsorptive powers
§ It
is used in dusting powders as a protective and lubricant, to prevent irritation
due to friction
§ It
also is used in medicated dusts and used widely in cosmetic applications. o The
bentonites are used as suspending agents
§ The
bentonites have gelling properties as well as ion-exchange properties and
detergent properties
o
Kaolins are used as clarifying agents and are
good excipients for inorganic salts
§ Kaolinsare
also used as intestinal adsorbents and protectives
§ Externally
they are used as dusting powders o Magnesium trisilicate is used as antacid
o
Magnesium trisilicate also is employed as a
suspending agent
o
Simethicone USP, a polymeric dimethylsiloxane,
is used as an antiflatulent in gastric bloating and in postoperative gaseous
distention in the gastrointestinal tract
o
Stannous fluoride (tin (II) fluoride) is applied
topically as a dental prophylactic o Various tin dioxide (tin(IV) oxide)
preparations are used externally for their germicidal effect, particularly
against staphylococcal organisms that are often resistant to other germicides
STEP 6: Key
Points (5 minutes)
Carbon
Family (Group 4A or 14) o Refers to Carbon (C), Silicon (Si) and
Germanium (Ge), Tin (Sn) and Lead (Pb).
Atoms
of this family have 4 valence electrons
Some
are non-metal (Carbon), metalloids (Silicon and Germanium), and metals (Tin and
Lead)
Elements
of this group also exhibit multiple oxidation states
The
Group 4A(14) elements are oxidized by halogens and oxygen
The
compounds of group four elements are used in pharmacy for preparation of
various Pharmaceuticals
STEP 7:
Evaluation (5 minutes)
What
are the group four elements?
What
are the physical characteristics of group four elements?
What
are the chemical characteristics of group four elements? What is the
importance of Group four elements?
References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6thed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rded.). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S &Lutfun, N. (2007).Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21sted.). Baltimore, MA. A Wolters Kluwer
Company.
Session 10: Characteristics and Uses of Group Five Elements in
Pharmacy
Total Session Time: 60 minutes
Prerequisites
None
Learning Tasks
By the end of this session students are expected to be able
to:
List
the group five elements
Describe
the physical characteristics of group five elements
Describe
the chemical characteristics of group five elements
Describe
the use of Group five elements in pharmacy
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Group Five Elements
|
|
3
|
10 minutes
|
Presentation
Buzzing
|
Physical Characteristics of Group Five
Elements
|
|
4
|
15 minutes
|
Presentation
Brainstorming
|
Chemical Characteristics of Group Five
Elements
|
|
5
|
15 minutes
|
Presentation
|
The Use of Group Five Elements in Pharmacy
|
|
6
|
05 minutes
|
Presentation
|
Key Points
|
|
7
|
05 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Group Five Elements (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are group five elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Nitrogen
Family (Group 5A or 15)
The
nitrogen family is named after the element that makes up 78% of our
atmosphere o Family
includes non-metals, metalloids and metals
o
Elements in this group are Nitrogen (N),
phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi)
o
Nitrogen and phosphorus are non-metals, arsenic
and antimony are metalloids, and bismuth is a metal
STEP 3:
Physical Characteristics of Group Five Elements (10 minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
are the physical characteristics of group five elements?
ALLOW few
pairs to respond and let other pairs to add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
Atoms
in the nitrogen family have 5 valence electrons
They
tend to share electrons when they bond
The
element phosphorus comes in two formswhite and red
Nitrogen
is a diatomic gas (N2) with a very low boiling point, due to its very weak intermolecular forces
Phosphorus
exists most commonly as tetrahedral P4 molecules o It has stronger dispersion forces than N2
Figure 10.1: Properties of group five elements
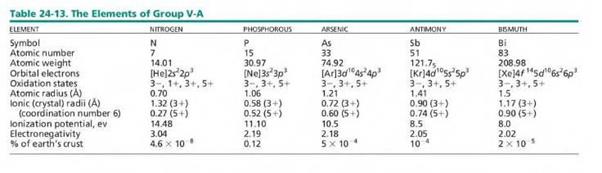
Source: Remington: The science and practice of
pharmacy (2015)
Arsenic
has a covalent network structure and has a high melting point
Antimony
also has a covalent network structure
Bismuth
has metallic bonding o Its melting point is lower than that of
As or Sb
The
higher elements in the group are metallic and lose electrons to form cations
Oxides
change from acidic to amphoteric to basic as you move down the group All Group 5A (15)
elements form gaseous hydrides with the formula 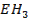
STEP 4:
Chemical Characteristics of Group Five Elements (15 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are the chemical characteristics of group five elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Nitrogen
reacts with hydrogen to form ammonia
Other
elements reacts with water or acid to form hydrides
Reacts with halogens to form halide (except N)
Their
halide reacts with water to form oxoacids (except N)
Nitrogen
forms a number of oxides (NO, N2O, NO2, N2O4,
and N2O5) o Only N2O5 is a
solid; the others are gases
Nitrogen
accept three electrons to form the nitride ion, N3+
Most
metallic nitrides example Li3N and Mg3N2) are
ionic compounds
Phosphorus
exists as P4 molecules
White
phosphorus burst into flames when exposed to oxygen in the air (is very active)
or it will
The
heads of matches contain the less active red phosphorus, which ignites from the
heat produced by friction when the match is struck
It
forms two solid oxides with the formulas P4O6 and P4O10
Their
oxides reacts with water to form oxoacids HNO3 and H3PO4

STEP 5: The
Use of Group Five Elements in Pharmacy (15 minutes)
Some
group five compounds have physiological function o A complex basic calcium phosphate
(hydroxyapatite) constitutes the main inorganic component of bones and
teeth
o
Phosphate has important roles in the metabolism
of various organic materials, such as carbohydrates
Most of their compounds are used in production
of pharmaceuticals o Nitrogen (I) Oxide (nitrous oxide) USP,
is used as an inhalatory general anesthetic.
o Sodium
Nitrite USP is used as an antidote to cyanide poisoning
o
The nitrate ion frequently is used as an anion
for medicinally active cations, such as silver nitrate and thiamine
mononitrate
o
The organic nitrites and sodium nitroprusside
are used to lower blood pressure
§ Nitric oxide (NO)
is paramagnetic molecule and is an important neurotransmitter produced by
neurons and other cells, causing responses such as vasodilation by acting as a
ligand for iron in a heme group with a resulting lowering of blood pressure
o
Tribasic calcium, magnesium, and aluminium
phosphates are used as gastric antacids o Monobasic alkali phosphates are
effective urinary acidifiers
o
Dibasic sodium phosphate is the active
ingredient in various saline cathartics and enemas
o
Phosphoric Acid NF, is used to form soluble
salts of insoluble medicinal bases o The
dihydrogen phosphate-monohydrogen phosphate system is a valuable buffer in
physiological ranges
o Hypophosphorous
Acid NF is an antioxidant, used primarily with iodide and iron (Il) salts o The radioactive isotope, 32P,
is employed therapeutically
STEP 6: Key
Points (5 minutes)
These
are Nitrogen Family (Group 5A or 15) o Refers to Nitrogen (N), phosphorus (P),
arsenic (As), antimony (Sb), and bismuth (Bi)
This
family includes non-metals, metalloids and metals Atoms in the
nitrogen share electrons when they bond
Their
oxides change from acidic to amphoteric to basic as you move down the group
All
Group 5A (15) elements form gaseous hydrides with the formula 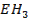
They
react with water or acid to form hydrides
Reacts
with halogens to form halide (except N)
Some
group five compounds have physiological function and most of their compounds
are used in production of pharmaceuticals
STEP 7:
Evaluation (5 minutes)
What
are the group five elements?
What
are the physical characteristics of group five elements?
What
are the chemical characteristics of group five elements? What are the uses
of Group Five elements?
References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6th ed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rd ed.). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S, & Lutfun, N. (2007). Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21st ed.). Baltimore, MA. A Wolters
Kluwer Company.
Session 11: Characteristics and Uses of Group Six
Elements in Pharmacy
Total Session Time: 60 minutes
Prerequisites
None
Learning Tasks
By the end of this session students are expected to be able
to:
List
the group six elements
Describe
the physical characteristics of group six elements
Describe
the chemical characteristics of group six elements
Describe
uses of Group six elements
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Group Six Elements
|
|
3
|
10 minutes
|
Presentation
Buzzing
|
Physical Characteristics of Group Six
Elements
|
|
4
|
10 minutes
|
Presentation
Brainstorming
|
Chemical Characteristics of Group Six
Elements
|
|
5
|
15 minutes
|
Presentation
|
Uses of Group Six Elements
in Pharmacy
|
|
6
|
05 minutes
|
Presentation
|
Key Points
|
|
7
|
10 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing
STEP 2:
Group Six Elements (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are group six elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
These are oxygen family (6A or 16) o Includes
oxygen (O), Sulphur (S), and selenium (Se), tellurium (Te) and polonium (Po).
STEP 3:
Physical Characteristics of Group Six Elements (10 minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
are the physical characteristics of group six elements?
ALLOW few
pairs to respond and let other pairs to add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
Physical characteristics of
group six elements include the following:
Oxygen,
Sulphur and selenium are non-metals
Tellurium
and polonium are metalloids
Oxygen is a diatomic gas
Elemental
Sulphur and selenium have the molecular formulas S8 and Se8
respectively Atoms
of this family have 6 valence electrons
Most
elements in this family share electrons when forming compounds
Oxygen
is the most abundant element in the earths crust o It
is extremely active and combines with almost all elements
The
heavier members of the group, tellurium and polonium, are both metalloids
Oxygen,
like nitrogen, occurs as a low-boiling diatomic gas, O2
Sulphur,
like phosphorus, occurs as a polyatomic molecular solid
Figure 11.1: Properties of group six elements
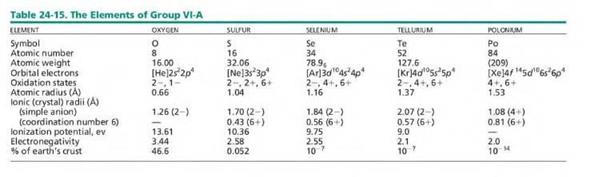
Source: Remington: The science and practice of
pharmacy (2015)
Selenium,
like arsenic, commonly occurs as a grey metalloid
Tellurium,
like antimony, displays network covalent bonding
Polonium,
like bismuth, has a metallic crystal structure
STEP 4:
Chemical Characteristics of Group Six Elements (10 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are the chemical characteristics of group six elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Chemical characteristics of group six elements are:
Polonium
is a radioactive element that is difficult to study in the laboratory
Oxygen has a tendency to accept two electrons to
form the oxide ion (O2-) in many ionic compounds
Sulphur,
selenium, and tellurium form negative anions (S2-, Se2-,
and Te2-)
The
elements in this group (especially oxygen) form a large number of molecular
compounds with non-metals
The
important compounds of Sulphur are SO2, SO3, and H2S
Sulphuric
acid is formed when Sulphur trioxide reacts with water
STEP 5: Uses
of Group Six Elements in Pharmacy (15 minutes)
Elements,
molecules and compounds of group six elements are used in preparation of
pharmaceuticals used in therapeutics and treatment
o
Oxygen USP is employed as a therapeutic gas in
the treatment of conditions involving hypoxia
o
Ozone, 03, an allotropic form of oxygen, is a
powerful oxidizing agent o Ozonized air (air treated to convert
some of its oxygen into ozone) is used in various disinfecting and bleaching
operations
o
Hydrogen peroxide as oxidizing agent is used in
various pharmaceutical preparations such as mouthwashes and disinfectants
§ Hydrogen
peroxide is prepared by the electrolysis of a concentrated solution of either
Sulphuric acid or ammonium sulphate
§ Hydrogen
peroxide is available as the 3, 6, 30, 70, and 90% solutions
§ It
is a powerful oxidant and higher concentration must not be used on the skin
§ Hydrogen
Peroxide Topical Solution USP (3%) solution is a mild, fast acting, oxidizing
germicide that will destroy most pathogenic bacteria
§ Hydrogen
peroxide, 6%, is the only common bleach mild enough for use on hair o Precipitated
Sulphur as ointment is used as the scabicide
o
Sulphur ointments and lotions are used in
dermatological applications as keratolytics o Elemental Sulphur also has fungicidal
action o Zinc
sulphate is used in management of diarrhoea
o
Anhydride of Sulphurous acid and its salts, the
sulphites are used in pharmaceutical practice as antioxidants and preservatives
o
Sodium Thiosulfate USP is used as an antidote
for cyanide poisoning
STEP 6: Key
Points (5 minutes)
Oxygen Family (6A or 16) o Refers
to oxygen (O), Sulphur (S), and selenium (Se), tellurium (Te) and polonium
(Po).
Some
elements are non-metals and others are metalloids
Atoms
of this family have 6 valence electrons
Most
elements in this family share electrons when forming compounds
Oxygen
is the most abundant element in the earths crust, extremely active and
combines with almost all elements
The
elements in this group (especially oxygen) form a large number of molecular compounds
with non-metals
Elements,
molecules and compounds of group six elements are used in preparation of
pharmaceuticals
STEP 7:
Evaluation (10 minutes)
What
are the group six elements?
What
are the physical characteristics of group six elements?
What
are the chemical characteristics of group six elements?
What
are the uses of Group six elements?
References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6thed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rded.). London: Pharmaceutical press.
Manning, P. (2008).Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S.,&Lutfun, N. (2007).Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21sted.). Baltimore, MA. A Wolters Kluwer
Company.
Session 12: Characteristics and Uses of Group Seven Elements in
Pharmacy
Total Session Time: 60 minutes
Prerequisites
None
Learning Tasks
By the end of this session students are expected to be able
to:
List the group seven elements
Describe the physical characteristics of group
seven elements
Describe the chemical characteristics of group
seven elements Describe
the use of Group seven elements in pharmacy
Resources Needed:
Flip charts, marker pens, and masking tape
Black/white board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Group Seven Elements
|
|
3
|
10 minutes
|
Presentation
Buzzing
|
Physical Characteristics of Group Seven
Elements
|
|
4
|
15 minutes
|
Presentation
Brainstorming
|
Chemical Characteristics of Group Seven
Elements
|
|
5
|
15 minutes
|
Presentation
|
The Use of Group Seven Elements in
Pharmacy
|
|
6
|
05 minutes
|
Presentation
|
Key Points
|
|
7
|
05 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Group Seven Elements (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are group seven elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
These are Halogen Family (Group 7A or 17) o These
elements are called halogens, which means salt-former
o
The elements in this family are fluorine (F),
chlorine (Cl), bromine (Br), iodine (I), and astatine (At)
o
Hydrogen may also be placed in this group as it
shares some features
STEP 3:
Physical Characteristics of Group Seven Elements (10 minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
are the physical characteristics of group seven elements?
ALLOW few
pairs to respond and let other pairs to add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
Have 7 valence electrons, which explain why they
are the most active non-metals.
They are never found free in nature
All the elements in Group 7 are non-metals
except for astatine, which is a radioactive metalloid
They exist as diatomic molecules (so that they
both have a full outer shell)
Fluorine and chlorine are liquid at room
temperature and bromine is a gas
The halogens have high ionization energies
They have high electron affinities
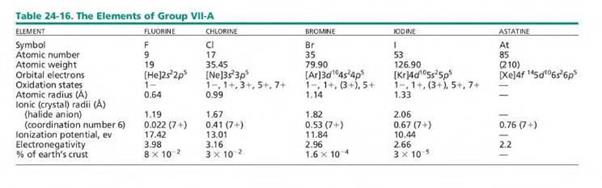
Figure 12.1: Properties of group seven elements
Source: Remington: The science and practice of
pharmacy (2015)
Anions derived from the halogens (F2,
Cl2, Br2, and I2) are called halides o They are isoelectronic with the noble
gases immediately to their right in the periodic table
o
F2 is isoelectronic with Ne and Cl2
with Ar
STEP 4:
Chemical Characteristics of Group Seven Elements (15 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are the chemical characteristics of group seven elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Halogen gains 1 electron to fill their outermost
energy level
All of the halogens form salts with sodium and
with the other alkali metals
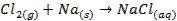
Reactivity decreases as you go down the group
(This is because the electrons are further away from the nucleus and so any
extra electrons arent attracted as much)
Fluorine is the most reactive and Iodine is the
least reactive of the four non-metals.
o
Fluorine is so reactive that it attacks water to
generate oxygen
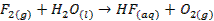
The halogens undergo disproportionation in water
The vast majority of the alkali metal halides
and alkaline earth metal halides are ionic compounds
The halogens also form many molecular compounds
among themselves (such as ICl and BrF3) and with non-metallic
elements in other groups (such as NF3, PCl5, and SF6)
The halogens react with hydrogen to form
hydrogen halides o Fluorine reacts explosively
o
Others react less violently
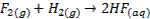
The hydrogen halides dissolve in water to form
hydrohalic acids.
Hydrofluoric acid (HF) is a weak acid (that is,
it is a weak electrolyte), but the other hydrohalic acids (HCl, HBr, and HI)
are all strong acids (strong electrolytes)
Hydrogen as halogen forms the hydride ion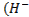 )
in ionic compounds such as NaH and
)
in ionic compounds such as NaH and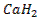 .
.
o
Ionic hydrides react with water to produce
hydrogen gas and the corresponding metal hydroxides

STEP 5: The
Use of Group Seven Elements in Pharmacy (15 minutes)
Physiological role
o
Group seven elements have a major physiological
role in human body
§ Fluorine
is an essential element and is present in the teeth and bone
§ Chlorine
is important for transportation of materials of the cell
§ Iodine
is essential for proper thyroid functioning
As a dental prophylactic
o
Fluoride has been included in various
preparations such as toothpastes essentially as a dental prophylaxis
Preparation of acidifying agents
o
Chlorine compounds particularly Hydrochloric
acid NF is a used for neutralizing, stabilizing, or solubilizing other
substances § In
diluted form, it is used as a gastric acidifier
Preparation of electrolyte replenisher o Sodium,
potassium, and calcium chlorides are employed in electrolyte replenisher o Potassium
or sodium iodide as sources of Iodine are essential for proper thyroid
functioning
Preparation of expectorants
o
Ammonium chloride is an expectorant and a
systemic acidifying agent
Preparation of Disinfectants, antiseptics and
sanitizing agents o Sodium Hypochlorite Solution USP
(Dakin's Solution) is an effective germicide, viricide, and deodorant because
of the oxidizing power of hypochlorous acid
§ Not suitable for
wounds because is too alkaline and concentrated
o Calcium
hypochlorite as bleaching powder is used as disinfectant o Povidone-Iodine and its dosage forms are
used as sanitizing agents
Diagnosis in medicine
o
The radioactive isotopes, 125I and 131I
have diagnostic and therapeutic applications o Iodine-131 (a 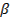 emitter with a half-life of 8 days) has been
used to test the activity of the thyroid gland
emitter with a half-life of 8 days) has been
used to test the activity of the thyroid gland
§ A
malfunctioning thyroid can be detected by giving the patient a drink of a
solution containing a known amount of Na131I and measuring the
radioactivity just above the thyroid to see if the iodine is absorbed at the
normal rate
o
Another radioactive isotope of iodine,
iodine-125 (a 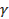 -ray
emitter), is used to image the thyroid gland
-ray
emitter), is used to image the thyroid gland
STEP 6: Key
Points (5 minutes)
These are Halogen Family (Group 7A or 17) o Refers
to fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At)
Have 7 valence electrons, which explain why they
are the most active non-metals They
are never found free in nature
All the elements in Group 17 are non-metals
except for astatine, which is a radioactive metalloid
They exist as diatomic molecules (so that they
both have a full outer shell) They
have high electron affinities
Anions derived from the halogens (F2,
Cl2, Br2, and I2) are called halides
Halogen gains 1 electron to fill their outermost
energy level
All of the halogens form salts with sodium and
with the other alkali metals
Their reactivity decreases as you go down the
group
The halogens undergo disproportion in water
The halogens form many molecular compounds among
themselves (such as ICl and BrF3) and with non metallic elements in
other groups (such as NF3, PCl5, and SF6)
The halogens react with hydrogen to form
hydrogen halides
The hydrogen halides dissolve in water to form
hydrohalic acids
The
elements and compounds made of group seven elements have various pharmaceutical
importance
STEP 7:
Evaluation (5 minutes)
What are the group seven elements?
What are the physical characteristics of group
seven elements?
What are the chemical characteristics of group
seven elements?
What is the importance of Group seven elements?
References
Chang, R.,
& Overby, J. (2011). General chemistry: The Essential Concepts (6th
ed.). New York, NY: The McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rd ed.). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S, & Lutfun, N. (2007). Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21st ed.). Baltimore, MA. A Wolters
Kluwer Company.
Session 13: Characteristics and Uses of Group Eight
Elements in Pharmacy
Total Session Time: 60 minutes
Prerequisites
None
Learning Tasks
By the end of this session students are expected to be able
to:
List
the group eight elements
Describe
the physical characteristics of group eight elements
Describe
the chemical characteristics of group eight elements Describe the uses
of group eight elements
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Group Eight Elements
|
|
3
|
15 minutes
|
Presentation
Buzzing
|
Physical Characteristics of Group Eight
Elements
|
|
4
|
10 minutes
|
Presentation
Brainstorming
|
Chemical Characteristics of Group Eight
Elements
|
|
5
|
10 minutes
|
Presentation
|
Uses of Group Eight
Elements in Pharmacy
|
|
6
|
05 minutes
|
Presentation
|
Key Points
|
|
7
|
10 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Group Eight Elements (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are group eight elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Noble gases (Group 8A or 18) o The
family of noble gases includes helium(He), neon(Ne), argon(Ar), krypton(Kr),
xenon(Xe), and radon(Rn)
STEP 3:
Physical Characteristics of Group Eight Elements (15minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
are the physical characteristics of group eight elements?
ALLOW few
pairs to respond and let other pairs to add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
Figure 13.1: Properties of group eight elements
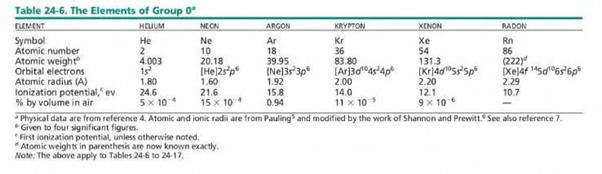
Source: Remington: The science and practice of
pharmacy (2015)
Found
in group 18 (the last column of the periodic table.)
Noble
Gases are colourless gases that are extremely un-reactive
All
the noble gases are found in small amounts in the earth's atmosphere
Noble
gases have a full valence shell
The
noble gases are the smallest elements in their respective periods, with the
highest ionization energies
Atomic
size increases down the group
Ionization
energy decreases down the group
Noble
gases have very low melting and boiling points
The
Group 8A ionization energies are among the highest of all elements, and these
gases have no tendency to accept extra electrons
STEP 4:
Chemical Characteristics of Group Eight Elements (10 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are the chemical characteristics of group eight elements?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
They
are inert o Inactive because outermost energy level
is full
o Do not react easily with other elements,
as they have a full outer shell
They do not readily combine with other elements
to form compounds Only
Kr, Xe, and Rn are known to form compounds
Xe
is the most reactive noble gas and exhibits all even oxidation states from +2
to +8
STEP 5: Uses
of Group Eight Elements (10 minutes)
Diagnostic
studies o 133Xe
is used for diagnostic studies both by inhalation and intravenous injection
Treatment
procedures o Radon
is used instead of radium in the treatment of certain types of cancer
§
Sealed tubes containing the gas are embedded in
the tissues to be treated
§
Both radium and radon emit alpha particles in
the first stage of their radioactive decay
STEP 6: Key
Points (5 minutes)
Noble gases (Group 8A or 18) o Refers
to helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn)
Noble
Gases are colourless gases that are extremely un-reactive.
The
noble gases are the smallest elements in their respective periods, with the
highest ionization energies
Only
Kr, Xe, and Rn are known to form compounds
Xe
is the most reactive noble gas and exhibits all even oxidation states from +2
to +8.
The
group eight elements are useful in pharmacy particularly in diagnostic studies
and treatment procedures
STEP 7:
Evaluation (10 minutes)
What
are the group eight elements?
What
are the physical characteristics of group eight elements?
What
are the chemical characteristics of group eight elements? What are the uses
of Group eight elements?
References
Chang,
R.,& Overby, J. (2011). General
chemistry: The Essential Concepts (6thed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rded.). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S & Lutfun, N. (2007).Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21sted.). Baltimore, MA. A Wolters Kluwer
Company.
Session 14: Characteristics and Uses of Actinides and
Lanthanides in Pharmacy
Total Session Time: 60 minutes
Prerequisites
None
Learning Tasks
By the end of this session students are expected to be able
to:
List
the actinides and lanthanides
Describe
the physical characteristics of actinides and lanthanides
Describe
the chemical characteristics of actinides and lanthanides Describe
the importance of actinides and lanthanides
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
10 minutes
|
Presentation
Brainstorming
|
Actinides and Lanthanides
|
|
3
|
10 minutes
|
Presentation Buzzing
|
Physical Characteristics of Actinides and
Lanthanides
|
|
4
|
10 minutes
|
Presentation
Brainstorming
|
Chemical Characteristics of Actinides and
Lanthanides
|
|
5
|
05 minutes
|
Presentation
|
The Use of Actinides and Lanthanides in
Pharmacy
|
|
6
|
10 minutes
|
Presentation
|
Key Points
|
|
7
|
10 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Actinides and Lanthanides (10 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are Actinides and Lanthanides?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Lanthanides
o Found
on period six of the table
o
They include: Cerium (Ce), Praseodymium (Pr),
Neodymium (Nd), Promethium (Pm),
Samarium (Sm), Europium (Eu),
Gadolinium (Tb), Terbium (Tb), Dysprosium(Dy),
Holmium
(Ho), Erbium (Er), Thulium (Tm), Ytterbium (Yb) and Lutetium (Lu)
Actinides
o Found
on period seven of the table
o They
include: Thorium (Th), Protactinium (Pa), Uranium (U), Neptunium (Np),
Plutonium (Pu), Americium (Am),
Curium (Cm), Berkelium (Bk), Californium (Cf),
Einsteinium (Es), Fermium (Fm),
Mendelevium (Md), Nobelium (No) and Lawrencium (Lr)
STEP 3:
Physical Characteristics of Actinides and Lanthanides (10 minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
are the physical characteristics of Actinides and Lanthanides?
ALLOW few
pairs to respond and let other pairs add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
Most
of the elements in the actinide series are synthetic or man-made
The
elements (sometimes called rare earths) are found together in nature and are
electropositive
Moving
from left to right across the period (increasing atomic number), the radius of each
lanthanide 3+ ion steadily decreases
They
have high melting points and boiling points
The
metals fill the seven orbitals of the 4f shells
The
lanthanides are soft metals that can be cut with a knife
The
elements are so similar that they are hard to separate when they occur in the
same ore, which they often do
Thorium,
protactinium, and uranium are the only actinides that now are found naturally
on Earth
Uranium
is found in Earths crust because its half-life is long4.5 billion years All other actinides
are synthetic elements
Synthetic
elements are made in laboratories and in nuclear reactors
STEP 4:
Chemical Characteristics of Actinides and Lanthanides (10 minutes)
Activity: Brainstorming (5
minutes)
Ask students to
brainstorm on the following question:
What
are the chemical characteristics of Actinides and Lanthanides?
ALLOW
few students to respond?
WRITE their
responses on the flip chart/ board
CLARIFY
and SUMMARISE by using the content
below
All actinides are radioactive o The nuclei of atoms of radioactive
elements are unstable and decay to form other elements
Lanthanides are: o Very reactive o React
with water to liberate hydrogen (H2); slowly in cold/quickly upon
heating
Lanthanides
commonly bind to water o React with H+ (dilute acid) to release H2
(rapidly at room temperature) o React in an exothermic reaction with H2
o Burn
easily in air o They are strong reducing agents o Their
compounds are generally ionic o At elevated temperatures, many rare
earths ignite and burn vigorously o Many rare earth compounds fluoresce
strongly under ultraviolet light o The magnetic moments of the lanthanide
and iron ions oppose each other o The lanthanides react readily with most
non-metals and form binaries on heating with most non-metals
STEP 5: The
Use of Actinides and Lanthanides in Pharmacy (5 minutes)
In production of radiopharmaceuticals o Radiopharmaceuticals
are radioactive drugs used for diagnosis or therapy in a tracer quantity with
no pharmacological effect
o All
the actinides are radioactive but have long half-life making them unsuitable to
be used directly in nuclear imaging
o Most
of them example uranium are decomposed to smaller fragments by nuclear fission
in the nuclear reactors
o
The radioactive elements with short half-life
are used to produce radiopharmaceuticals
STEP 6: Key
Points (10 minutes)
Lanthanides are found on period six of the table
Actinides
are found on period seven of the table
Most
of the elements in the actinide series are synthetic or man-made
Actinides
have high melting points and boiling points
All
other actinides are synthetic elements
All
the actinides are radioactive
The
lanthanides are soft metals that can be cut with a knife
The
elements are so similar that they are hard to separate when they occur in the
same ore, which they often do
Thorium,
protactinium, and uranium are the only actinides that now are found naturally
on Earth
Lanthanides
are very reactive and they are strong reducing agents
Many
rare earth compounds fluoresce strongly under ultraviolet light
The
lanthanides react readily with most non-metals and form binaries on heating
with most non-metals
STEP 7:
Evaluation (10 minutes)
What
are the rare earth elements?
What
are the physical characteristics of rare earth elements?
What
are the chemical characteristics of rare earth elements?
What is the importance of rare earth elements?
References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6thed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rded.). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S.,& Lutfun, N. (2007).Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21sted.). Baltimore, MA. A Wolters Kluwer
Company.
Session 15: Description of Compounds
Total Session Time: 120
minutes + 2 hours of Assignment and Tutorial
Prerequisites
None
Learning Tasks
By the end of this session students are expected to be able
to:
Define
matter
Classify
matter
Define
the term compound
Name
simple ionic compounds
Describe
complex compounds
Describe
the characteristics of compounds
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
Handout
16.1: Simple Ions and Compound Ions
Handout
16.2: Common Ligands
Handout
16.3: Names of Common Ligands in Coordination Compounds
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Definition of Matter
|
|
3
|
05 minutes
|
Presentation
|
Classification of Matter
|
|
4
|
05 minutes
|
Presentation
|
Definition of Compound
|
|
5
|
40 minutes
|
Presentation Small group discussion
|
Naming Simple Ionic
Compound
|
|
6
|
30 minutes
|
Presentation
|
Complex Compounds
|
|
7
|
10 minutes
|
Presentation
|
Characteristics of
Compounds
|
|
8
|
05 minutes
|
Presentation
|
Key Points
|
|
9
|
05 minutes
|
Presentation
|
Evaluation
|
|
10
|
10 minutes
|
Presentation
|
Assignment
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Definition of Matter (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
is matter?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Matter
o Refers to anything that has got mass
and occupies space
STEP 3:
Classification of Matter (5 minutes)
Matter
may be classified physically or chemically
o Physically
§
Solid, liquid and gas o Chemically
§
Elements, compounds and mixture
STEP 4:
Definition of Compound (5 minutes)
Compounds
o Refers
to two or more elements chemically combined to form a new substance with new
properties
o Example
Hydrogen gas burns in oxygen gas to form water, a compound whose properties are
distinctly different from those of the starting materials
§ Water
is made up of two parts of hydrogen and one part of oxygen o The composition does not change,
regardless of the place water comes from
o Can
only be separated only by chemical means into their pure components
STEP 5:
Naming Simple Ionic Compounds (40 minutes)
|
Activity: Small Group Discussion ( 30
minutes)
DIVIDE
students into small manageable groups
ASK students
to discuss on the following question
What
are the procedures for naming ionic compounds?
ALLOW students
to discuss for 15 minutes
ALLOW few
groups to present and the rest to add points
not mentioned
CLARIFY and SUMMARIZE
by using the contents below
|
The following are the procedures of naming Simple Ionic
Compounds:
The upper case (capital) letters must be clearly
written as capital letters, lower case (small) letters must be written clearly
as small letters, subscript numbers (small numbers after a symbol) must be
written accurately and clearly The
name of an ionic compound has two parts:
o The
first part is the positive ion, usually a metal, but may also be ammonium, a
positive compound ion containing nitrogen and hydrogen
o The
second part of the name is the negative ion, either the name of a non-metal
with the end of its name changed to -ide, or the name of a negative compound
ion Table 16.1: Parts of ionic compound
|
Name of compound
|
Positive ion
|
Negative
ion
|
|
Calcium iodide
|
calcium, 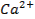
|
iodide, 
|
|
Copper phosphate
|
copper, 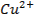
|
phosphate, 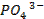
|
|
Aluminum sulfate
|
aluminum, 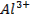
|
sulfate, 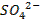
|
|
Ammonium chloride
|
ammonium, 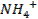
|
chloride, 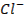
|
An
acid contains hydrogen joined with a negative ion, and has "acid" as
the second word of its name
o The
first word is usually the name of the negative ion, with the end of its name
changed: -ate changes to ic and -ite changes to -ous
Table 16.2: Names of acidic compound
|
Name of acid
|
Positive ion
|
Negative
ion
|
|
Nitric acid
|
Hydrogen, 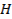
|
Nitrate, 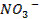
|
|
Carbonic acid
|
Hydrogen, 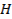
|
Carbonate, 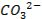
|
|
Sulfurous acid
|
Hydrogen, 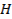
|
Sulphite, 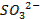
|
The names of negative ions end in -ide, -ite, or
-ate
o The
ending "-ide" means that the ion contains only one atom (except
hydroxide, OH, and cyanide, CN)
o The
endings "-ite" and "-ate" indicate that the ion is a
compound ion, with "-ite" ions containing less oxygen than
"-ate" ions
o If
only one compound ion with oxygen exists for a particular element, the
"-ate" ending is used
o Some
compounds have special prefixes, such as "per-", "hypo-",
and "thio-"
§ "Per-"
means even more oxygen than "-ate"
§ "hypo-"
means less oxygen than "-ite"
§ "Thio-"
means some sulfur is present instead of oxygen Table
16.3: Names of negative ions
|
Sulphide, 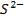
|
|
Sulfite, 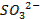
|
Sulphate, 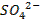
|
|
|
Nitride, 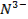
|
|
Nitrite, 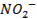
|
Nitrate,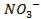
|
|
|
|
|
|
Carbonate,
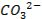
|
|
|
Phosphide, 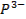
|
|
Phosphite,
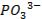
|
Phosphate,
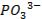
|
|
|
Chloride, 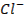
|
Hypochlorite,
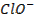
|
Chlorite,
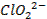
|
Chlorate 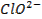
|
perchlorate,
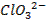
|
Formulae
are written by the following steps: o Identify the ions indicated in the
name o Check the charge values on the ions
§ Positive and negative values must balance, so the numbers of
positive and negative ions used must be chosen so that positive and negative
charges cancel out
o Combine
the ions into a single formula, leaving out charge values
§ The
number of each kind of ion used in the formula is shown by a subscript (small
number at the bottom after the symbol of each ion involved), except that the
number 1 is not shown
§
If there is two or more of a compound ion, its
formula should be enclosed in brackets, with the subscript outside
Refer students to Handout 16.1: Simple Ions and Compound Ions for further reading
STEP 6:
Complex Compounds (30 minutes)
Theory of Complex Compounds
Most
elements exhibit two types of valence: primary valence and secondary
valence.
Primary
valence corresponds to the oxidation number and secondary valence to the
coordination number of the element
Most,
but not all of the metals in coordination compounds are transition metals
The molecules or ions that surround the metal in
a complex ion are called ligands
Refer students to Handout 16.2: Common Ligands for further reading
The
interactions between a metal atom and the ligands can be thought of as Lewis
acidbase reactions
Lewis
base is a substance capable of donating one or more electron pairs o Every ligand has at least one unshared
pair of valence electrons
A transition metal atom (in either its neutral
or positively charged state) acts as a Lewis acid, accepting (and sharing)
pairs of electrons from the Lewis bases o The
metal-ligand bonds are usually coordinate covalent bonds
The
atom in a ligand that is bound directly to the metal atom is known as the donor
atom. o Nitrogen is the donor atom in the  complex ion
complex ion
The
coordination number in coordination compounds is the number of donor atoms
surrounding the central metal atom in a complex ion
o  The
coordination number of is 2 o The coordination number of is 4
The
coordination number of is 2 o The coordination number of is 4
o The
coordination number of is 6
Depending on the number of donor atoms present,
ligands are classified as monodentate, bidentate, or polydentate o  are monodentate ligands with only one donor
atom each. o One
bidentate ligand is ethylenediamine (sometimes abbreviated en) o Bidentate
and polydentate ligands are also called chelating agents because of their
ability to hold the metal atom like a claw
are monodentate ligands with only one donor
atom each. o One
bidentate ligand is ethylenediamine (sometimes abbreviated en) o Bidentate
and polydentate ligands are also called chelating agents because of their
ability to hold the metal atom like a claw
o Ethylenediamine
tetraacetate ion (EDTA), a polydentate ligand used to treat metal
poisoning
§ Six
donor atoms enable EDTA to form a very stable complex ion with lead, which is
removed from the blood and excreted from the body
§ EDTA
is also used to clean up spills of radioactive metals
Oxidation Number of Metals in Coordination
Compounds o The net charge of a complex ion is the sum
of the charges on the central metal atom and its surrounding ligands
o In
the 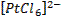 ion, for example, each chloride ion has an
oxidation number of -1, so the oxidation number of Pt must be +4
ion, for example, each chloride ion has an
oxidation number of -1, so the oxidation number of Pt must be +4
o
If the ligands do not bear net charges, the
oxidation number of the metal is equal to the charge of the complex ion
o In
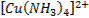 each NH3 is neutral, so the
oxidation number of Cu is +2
each NH3 is neutral, so the
oxidation number of Cu is +2
The Rules for Naming Coordination
Compounds
The
cation is named before the anion, as in other ionic compounds.
 The
rule holds regardless of whether the complex ion bears a net positive or a
negative charge o In
the and
compound, we name the K+ and
The
rule holds regardless of whether the complex ion bears a net positive or a
negative charge o In
the and
compound, we name the K+ and
cations first, respectively
Within
a complex ion, the ligands are named first, in alphabetical order, and the
metal ion is named last
The
names of anionic ligands end with the letter o, where as a neutral ligand is
usually called by the name of the molecule
o The
exceptions are H2O (aqua), CO (carbonyl), and NH3
(ammine)
Refer students to Handout 16.3: Names of Common Ligands in Coordination Compounds for
further reading
When
several ligands of a particular kind are present, we use the Greek prefixes
di-, tri-, tetra-, penta-, and hexa- to name them
o The
ligands in the cation 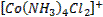 are tetraamminedichloro o The prefixes are ignored when
alphabetizing ligands
are tetraamminedichloro o The prefixes are ignored when
alphabetizing ligands
o If
the ligand itself contains a Greek prefix, we use the prefixes bis (2), tris
(3), and tetrakis (4) to indicate the number of ligands present
§ The
ligand ethylenediamine already contains di; therefore, if two such ligands are
present the name is bis (ethylenediamine)
The
oxidation number of the metal is written in Roman numerals following the name
of the metal
o  For
example, the Roman numeral III is used to indicate the +3 oxidation state of
chromium in which is called tetraamminedichloro chromium
(III) ion
For
example, the Roman numeral III is used to indicate the +3 oxidation state of
chromium in which is called tetraamminedichloro chromium
(III) ion
If
the complex is an anion, its name ends in -ate.
o
For example, in the anion 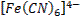 is called hexacyanoferrate (II) ion
is called hexacyanoferrate (II) ion
Coordination Compounds in Living
Systems
Coordination
compounds play many roles in animals and plants
They
are essential in the storage and transport of oxygen, as electron transfer
agents, as catalysts, and in photosynthesis
Example are cisplatin (cis-diamminedichloro
platinum (II), cis[Pt(NH3)2Cl2] ) an
anticancer drug and Hemoglobin which functions as an oxygen carrier for
metabolic processes
STEP 7:
Characteristics of Compounds (10 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are the characteristics of compounds?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Formed
by chemical combination of elements
Can
be broken into simpler substances by chemical means
Inorganic
compounds usually dont contain Carbon, generally come from the earth and are
generally simple molecules o Example are common table salt, water,
hydrochloric acid and carbon dioxide
Organic
compounds always contain C & H (usually O, N, sometimes S & P )
originate in organisms and generally complex molecules o Examples
of organic compounds are carbohydrates, lipids, proteins and nucleic acids (DNA
and RNA).
STEP 8: Key
Points (5 minutes)
Matter
o Refers
to anything that has got mass and occupies space
Matter
may be classified physically or chemically
Compounds
o Refers
to two or more elements chemically combined to form a new substance with new
properties
Inorganic
compounds usually dont contain Carbon, generally come from the earth and are
generally simple molecules
Organic
compounds always contain C & H (usually O, N, sometimes S & P )
originate in organisms and generally complex molecules
Compounds
that contain complex ions are called coordination compounds. Complex
ions consist of a metal ion surrounded by ligands
Coordination
compounds occur in nature and are used as therapeutic drugs
STEP 9:
Evaluation (5 minutes)
What
is matter?
How
do we classify matter?
What
is a compound?
What
are complex compounds?
How
do we name compounds?
What
are the characteristics of compounds?

References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6th ed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rd Ed). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S & Lutfun, N. (2007). Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21st ed.). Baltimore, MA. A Wolters
Kluwer Company.

Session 16: Description of Mixtures
Total Session Time: 60
minutes + 2 hours of Assignment and Tutorial
Prerequisites
Session
4
Learning Tasks
By the end of this session students are expected to be able
to:
Define
the term mixture
List
methods of separating mixtures Describe the
characteristics of mixtures
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
05 minutes
|
Presentation
|
Definition of Mixture
|
|
3
|
15 minutes
|
Presentation
Buzzing
|
Characteristics of Mixtures
|
|
4
|
15 minutes
|
Presentation
Brainstorming
|
Methods of Separating
Mixtures
|
|
5
|
05 minutes
|
Presentation
|
Key Points
|
|
6
|
05 minutes
|
Presentation
|
Evaluation
|
|
7
|
10 minutes
|
Presentation
|
Assignment
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Definition of Mixture (5 minutes)
Mixture o Refers to two or more substances that
are physically combined with each other and can be separated by physical means
o
Can either be homogeneous or heterogeneous
o
The mixture with uniform composition throughout
is a homogeneous mixture o Mixture
with non-uniform composition is a heterogeneous mixture o Any mixture, whether homogeneous or
heterogeneous, can be created and then separated by physical means into pure
components without changing the identities of the components
§ Eg,
syrup is a mixture of sugar and water; sugar can be recovered from syrup by
heating the syrup and evaporating it to dryness.
§ Water
can be obtained by condensing the water vapour.
o
After separation, the components of the mixture
will have the same composition and properties as before.
STEP 3:
Characteristics of Mixtures (15 minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
are the characteristics of mixtures?
ALLOW few
pairs to respond and let other pairs to add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
Following are the characteristics of mixtures:
Formed
by physical combination of components
Mixtures do not have fixed properties o Their properties depend on the nature of
their components and the ratios in which they are combined o Mixtures
do not have constant composition
In
mixtures, no new substance is formed o Substances
retain their distinct identities
o
The properties of a mixture are the same as the
properties of its constituents
The
constituents of a mixture can be separated easily by physical methods
Homogeneous
mixtures look the same throughout o Have one phase and uniform composition
Heterogeneous mixtures have more than one phase and
have a non-uniform composition
STEP 4:
Methods of Separating Mixtures (15 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
are the methods used to separate mixtures?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Following are the methods for separating mixtures:
Distillation
Fractional
distillation
Magnet
Filter
Decant
Evaporation
Centrifuge
Chromatography
STEP 5: Key
Points (5 minutes)
Mixture o Refers to two or more substances that
are physically combined with each other and can be separated by physical means
Mixtures
can be separated by Distillation, Fractional distillation, Magnet, Filter,
Decantation, Evaporation, Centrifuge and Chromatography
STEP 6:
Evaluation (5 minutes)
What
is mixture?
What
are the characteristics of mixtures?
What are the methods used to separate mixture?
STEP 7:
Assignment (10 minutes)
|
Activity: Take home Assignment (10 minutes)
DIVIDE
students in groups or individual.
ASK the
students to work on the following assignment
What
are the differences between mixtures and compounds?
ALLOCATE time
for students to do the assignment and submit
REFER students to recommended references
|
References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6th ed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rd Ed). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S & Lutfun, N. (2007). Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21st ed.). Baltimore, MA. A Wolters
Kluwer Company.
Session 17: Introduction to Physical and Chemical
Properties of Drugs
Total Session Time: 60 minutes
Prerequisites
None
Learning Tasks
By the end of this session students are expected to be able
to:
Define
physical and chemical characteristics of drug
List
the physical and chemical characteristics of drug
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
10 minutes
|
Presentation
|
Definition of Physical and Chemical
Characteristics of a Drug
|
|
3
|
30 minutes
|
Presentation Small group discussion
|
Physical and Chemical Characteristics of Drugs
|
|
4
|
05 minutes
|
Presentation
|
Key Points
|
|
5
|
10 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Definition of Physical and Chemical Characteristics of a drug
(10 minutes)
Physical
characteristics of a drug o Refers to characteristics of a drug that
can be observed or measured without changing the composition or identity of the
drug
o
Can be easily studied
Chemical property of a drug o Refers
to the characteristics of a drug that have the ability to produce a change in
the composition of the drug
o
Become evident during a chemical reaction o Cannot
be determined by viewing or touching
STEP 3:
Physical and chemical properties of drugs (30 minutes)
|
Activity: Small Group Discussion ( 30
minutes)
DIVIDE
students into small manageable groups
ASK students
to discuss on the following question
What
are the physical and chemical characteristics of the drug?
ALLOW students
to discuss for 15 minutes
ALLOW few
groups to present and the rest to add points
not mentioned
CLARIFY and SUMMARIZE
by using the contents below
|
Following are the chemical characteristics of
drugs o Toxicity o Chemical stability
Following
are the physical characteristics of drugs
o Physical
stability o Physical
state o Melting
point and boiling point o Organoleptic character (Color, taste)
o
Flow rate o Fluidity
o
Partition coefficient and pKa
o
Hardness o Solubility o Dissolution
o Ionization
o Velocity
o Viscosity
o Particle
size and surface area o Polymorphism
STEP 4: Key
Points (5 minutes)
Physical
characteristics of a drug o Refers to characteristics of a drug that
can be observed or measured without changing the composition of the drug
Chemical property of a drug o Refers
to the characteristics of a drug that have the ability to produce a change in
the composition of the drug
STEP 5:
Evaluation (10 minutes)
What
is the meaning of physical characteristics of drug?
What
is the meaning of chemical characteristics of drug?
State
the physical and chemical characteristics of drug
References
Donald, C.
(2008). Essentials of pharmaceutical
chemistry (3rd ed.). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S., & Lutfun, N. (2007). Chemistry for pharmacy students: General,
Organic and Natural Product Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21st ed.). Baltimore, MA. A Wolters
Kluwer Company.
Session 18: Introduction to Acids and their Importance in
Pharmacy
Total Session Time: 60 minutes
Prerequisites
None
Learning Tasks
By the end of this session
students are expected to be able to: Define
acid
Classify
acids
List
the distinctive properties of acids
Describe
the general properties of acids
Describe
the importance of acid in pharmacy
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Definition of Acid
|
|
3
|
05 minutes
|
Presentation
|
Classification of Acids
|
|
4
|
05 minutes
|
Presentation
|
Distinctive Properties of
Acids
|
|
5
|
10 minutes
|
Presentation
Buzzing
|
General Properties of
Acids
|
|
6
|
15 minutes
|
Presentation
|
Importance of Acid in
Pharmacy
|
|
7
|
05 minutes
|
Presentation
|
Key Points
|
|
8
|
10 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Definition of Acid (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
is an acid?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Arrhenius
definition of acid o A substance that produce H+ only as a
positively charged ion
§ Example
HCL, HBr, H2SO4 o Apply only in aqueous media (water)
Lewis
definition of acid o Any
species capable of accepting a pair of electrons (electron acceptor).
§ Examples: H+, and metal cations, molecules such as BF3
with incomplete octets, and ones such as SiF4 where octet expansion
is possible
Brψnsted
definition of Acid o A
proton donor
STEP 3:
Classification of Acids (5 minutes)
Strong
acid o The acid that ionizes completely
§ Example
HCl, H2SO4 and HNO3
Weak
acid o The acid that ionizes partially
§ Example
CH3COOH
STEP 4:
Distinctive Properties of an Acid (5 minutes)
Acids
taste sour
Aqueous
solutions of all Arrhenius acids contain hydrogen ions
Acids
have a pH of less than 7
Acids
turn phenolphthalein COLOURLESS and litmus RED
STEP 5:
General Properties of Acids (10 minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
are the general characteristics of an acid?
ALLOW few
pairs to respond and let other pairs to add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
Physical Properties of Acids o Acids
taste sour o Acids
have a pH of less than 7 o Acids are harmful to living cells
o
Aqueous solutions of all acids contain hydrogen
ions o Acid
turns blue litmus red o Strong acids are corrosive
o
Aqueous acid solutions conduct electricity
Chemical Properties of Acid o Metals
above copper in the reactivity series react with acids, giving off hydrogen
gas, forming a salt
o
Reacts with bases to form salts (neutralization
reaction)
o
Reacts with metal carbonates to produce salt,
water and carbon dioxide gas (effervescence is observed)
STEP 6:
Importance of Acid in Pharmacy (15 minutes)
Preparation
of analgesics o Example
Salicylic acid (active ingredient of Asprin)
Improve
solubility of drugs o Help in dissolving insoluble medicines
containing compounds with a functional group capable of acting as a strong base
§ Example Lidocaine Hydrochloride Injection USP prepared by
reacting lidocaine with hydrochloric acid; the diethyl amino group is a stronger
base than either the water molecule or the chloride ion and Lidocaine goes into
solution as a cation making it more soluble
Preparation
of skin remedies as keratolytic (soften the outer layer of skin) o Example salicylic acid
Used
as reagents for analytical procedures
As
buffer systems to maintain the pH of a medicinal agent at an optimal value
Preparation of effervescent mixtures (a
medicinal dosage form sometimes used to render a medicinal more palatable for
oral administration) o Example Solid acids such as citric acid,
tartaric acid, or sodium dihydrogen phosphate
Preparation
of germicides
o Example
Hypochlorous acid used in preparation of Sodium Hypochlorite, others are boric
Acid and Sodium Borate
STEP 7: Key
Points (5 minutes)
Acid o A
substance that produce H+ only as a positively charged ion o Any
species capable of accepting a pair of electrons (electron acceptor) o A
proton donor
Acids
can be categorized as strong acids or weak acids o They have a pH of less than 7, taste sour,
turn litmus paper red Most of the acids are used in Preparation of skin
remedies as keratolytic (soften the outer layer of skin), Preparation of
analgesics, Preparation of germicides
STEP 8:
Evaluation (10 minutes)
What
is Acid?
How
do we classify acids?
What
are the properties of acid?
What
is the importance of acid in pharmacy?
References
Donald, C.
(2008). Essentials of pharmaceutical
chemistry (3rd ed.). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S, & Lutfun, N. (2007). Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21st ed.). Baltimore, MA. A Wolters
Kluwer Company.
Session 19: Introduction to Bases and their Importance in Pharmacy
Total Session Time: 60 minutes
Prerequisites
None
Learning Tasks
By the end of this session
students are expected to be able to: Define
a base
Categorize
bases
List
the general properties of bases
List
the distinctive properties of bases
Describe
the importance of bases in pharmacy
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning Tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Definition of a base
|
|
3
|
05 minutes
|
Presentation
|
Categories of Bases
|
|
4
|
10 minutes
|
Presentation
Buzzing
|
General Properties of Bases
|
|
5
|
05 minutes
|
Presentation
|
Distinctive Properties of Bases
|
|
6
|
15 minutes
|
Presentation
|
Importance of Bases in Pharmacy
|
|
7
|
05 minutes
|
Presentation
|
Key Points
|
|
8
|
10 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Definition of Bases (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
is a Base?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Arrhenius
base o Base
is a substance that produce OH- only as a negatively charged ion
§ Example
NaOH, Mg(OH)2 o Apply only in aqueous media (water)
Lewis
base o Base
is any species with a pair of non-bonding electrons available for donation
(electron donor)
§ Examples
molecules such as NH3, and anions such as F-
Brψnsted
Base o A
proton acceptor
STEP 3:
Categories of Bases (5 minutes)
Strong
Base o The base that ionize completely
§ Examples
NaOH and KOH
Weak
Base
o
The base that ionize partially
§ Examples
NH3
STEP 4:
General Characteristics of Bases (10 minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
are the general characteristics of Bases?
ALLOW few
pairs to respond and let other pairs to add points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
Physical
Properties of Bases o Bases have a pH of more than 7 o Dilute
solutions of bases taste bitter
o
Bases turn phenolphthalein PINK and litmus
BLUE o Aqueous solutions of all alkalis contain
hydroxide ion o Bases feel slippery; for example, soaps,
which contain bases o Aqueous base solutions conduct
electricity
Chemical Properties of Bases o Bases
react with fats to form soap and glycerol (saponification) o Reacts
with acids to form salts (neutralization reaction)
o
React with Ammonium salts to produce ammonia
detected by its pungent odour
(strong smell) and by turning damp red
litmus blue o Alkali's
are used to produce the insoluble hydroxide precipitates of many metal ions
from their soluble salt solutions
STEP 5:
Distinctive Characteristics of Base (5 minutes)
Bases
have a pH of more than 7
Dilute
solutions of bases taste bitter
Bases
turn phenolphthalein PINK and litmus BLUE
Aqueous
solutions of all Arrhenius alkalis contain hydroxide ion Soapy touch
Bases
react with fats to form soap and glycerol (saponification)
STEP 6: Importance
of Bases in Pharmacy (15 minutes)
Improve
solubility of drugs o Help in dissolving insoluble medicinal
containing compounds with a functional group capable of acting as a strong acid
o
Niacin Injection USP prepared by reacting niacin
with either sodium carbonate or sodium hydroxide; the carboxyl group loses its
proton to the carbonate or hydroxyl ion and the niacin goes into solution as an
anion making it more soluble
Preparation
of effervescent mixtures (a medicinal dosage form sometimes used to render a
medicinal more palatable for oral administration) o Sodium bicarbonate is used as the carbon
dioxide source
Development
of gastric antacids o Example
magnesium antacids (Magnesium Hydroxide, Milk of Magnesia, Magnesia
Tablets,
Alumina and Magnesia Oral Suspension (and Tablets) and Magnesium
Trisilicate) and Aluminium
antacids (Aluminium Hydroxide Gel, Dried Aluminum
Hydroxide
Gel (and Capsules and Tablets), Aluminum Phosphate Gel, Alumina,
Magnesia, and Calcium Carbonate
Oral Suspension (and Tablets), Alumina and Magnesium Trisilicate Oral
Suspension (and Tablets)
As
systemic alkalizers and acidifiers o Example Sodium Bicarbonate USP and
Potassium Bicarbonate USP
STEP 7: Key
Points (5 minutes)
Base
o
A substance that produce OH- only as a
negatively charged ion
Bases
can be categorized as strong base or weak base
Bases
have a pH of more than 7
Bases
turn phenolphthalein PINK and litmus BLUE
o Bases
have importance in Pharmacy; Improve solubility of drugs and are used
inevelopment of gastric antacids
STEP 8:
Evaluation (10 minutes)
What
is a base?
What
are the properties of a base?
What
is the importance of a base in pharmacy?
References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6th ed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rd Ed). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S & Lutfun, N. (2007). Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21st ed.). Baltimore, MA. A Wolters
Kluwer Company.
Session 20: Introduction to Salts and their Importance in
Pharmacy
Total Session Time: 60
minutes + 2 hours assignment
Prerequisites
None
Learning Tasks
By the end of this session
students are expected to be able to: Define
salt
Categorize salts
Describe the general properties of salts
Describe the importance of salts in pharmacy
Resources Needed:
Flip charts, marker pens, and masking tape
Black/white board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
05 minutes
|
Presentation
Brainstorming
|
Definition of Salt
|
|
3
|
05 minutes
|
Presentation
|
Categories of Salts
|
|
4
|
10 minutes
|
Presentation
Buzzing
|
General Properties of Salts
|
|
5
|
15 minutes
|
Presentation
|
Importance of Salts in
Pharmacy
|
|
6
|
05 minutes
|
Presentation
|
Key Points
|
|
7
|
05 minutes
|
Presentation
|
Evaluation
|
|
8
|
10 minutes
|
Presentation
|
Assignment
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning Tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Definition of Salt (5 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
is salt?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
Salt o A compound that results from the
neutralization reaction of an acid and a base
o Composed
of related number of cations (positively charged ions) and anions
(negatively charged ions) to
make an electrically neutral product
STEP 3:
Categories of Salt (5 minutes)
Normal Salts o Normal salts are formed when all the
replaceable hydrogen ions in the acid have been completely replaced by metallic
ions
o
Normal salts are neutral to litmus paper
o
Example NaCl and ZnSO4
Acidic salt o Acidic salts are formed when replaceable
hydrogen ions in acids are only partially replaced by a metal
o
Acid salts are produced only by acids containing
more than one replaceable hydrogen ion
o
An acid salt will turn blue litmus red
o
In the presence of excess metallic ions an acid
salt will be converted into a normal salt as its replaceable hydrogen ions
become replaced
o
Example NaH2PO4, Na2HPO and KHSO4
Basic salt o Basic salts contain the hydroxide ion,
OH-
o
They are formed when there is insufficient
supply of acid for the complete neutralization of the base
o
A basic salt will turn red litmus blue and will
react with excess acid to form normal salt
o
Example Zn(OH)Cl and Mg(OH)NO3
STEP 4:
General Properties of Salts (10 minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
are the general characteristics of salts?
ALLOW few
pairs to respond and let other pairs to add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
Nearly all ionic compounds are crystalline
solids at room temperature
Better solubility and dissolution rates
Conduct electricity when molten or dissolved in
water o Solid ionic compounds do not conduct
electricity because in order for a substance to conduct electricity it must
have charged particles that can move freely
o
Ions in ionic compounds cannot move very much,
except to vibrate
Have an overall net charge o They are electrically neutral because
the amount of positive charge is equal to the amount of negative charge
Higher melting and boiling points compared to other
types of compounds (covalent compounds)
o
The ions in an ionic compound form strong bonds
with a number of different ions due to their arrangement into crystalline
structures
Hard and brittle because their ions are arranged
into unit cells which form layers. o As long as the layers stay aligned, the
ionic compound is hard o If
one layer is shifted, like charges will be next to one another o The repulsive forces between like ions
causes the layers to break apart
STEP 5:
Importance of Salts in Pharmacy (15 minutes)
They act as
systemic acidifiers o Example Ammonium Chloride USP, Monobasic
Sodium Phosphate USP, and Calcium Chloride USP
Used in preparation of parenteral infusions
intended to supply electrolytes, water, and carbohydrates as nutrients o Example
chloride and lactate salts in preparation of Normal saline (NS) injection,
Dextrose Normal saline (DNS)
injection and Lactated Ringer's (RL)
Used in preparation of Oral Rehydration Salts
(ORS) o A
dry mixture of sodium chloride, sodium bicarbonate (or sodium citrate),
potassium chloride, and dextrose to be dissolved and used to treat chronic
diarrhoea.
Used in preparation of mineral supplements o Example Mineral Capsules and Mineral
Tablets containing potassium, calcium, magnesium, phosphorous, zinc, iron,
manganese and iodine
Used in preparation of germicides o Salts
such as Silver Nitrate and Zinc Chloride
Used in preparation of astringents o Salts
such as Aluminium Chloride hydrate and aluminium
Used in preparation of skin protectives o Zinc Stearate (all USP) is used for
their protective and slightly astringent properties
Calamine is the calcined native zinc oxide ore o The iron oxide impurity gives calamine a
flesh colour that is cosmetically more appealing
o
Zinc stearate, a mixture of fatty acid zinc
soaps, has an unctuous feel o White
Lotion USP is used for its astringent and protective powers
Used in preparation of anticonvulsants o Example Magnesium Sulphate Injection USP
Used in preparation of expectorants o Example Ammonium chloride
Used in preparation of laxatives, enemas and
irrigation solutions o Example
magnesium sulphate and citrate of magnesia
Used as radiopaque and imaging agents o Radiopaque compounds are capable of
interfering with the passage of x-rays o Example Barium Sulphates are used for
studies of the intestinal tract
STEP 6: Key
Points (5 minutes)
Salt refers to a compound which results from the
neutralization reaction of an acid and a base
Salt can be categorized as normal Salt, acidic
salt or basic salt
They conduct electricity
They are used in; o Preparation of Oral Rehydration Salts
(ORS) o Preparation
of mineral supplements. o Preparation
of germicides o Preparation of astringents o Preparation
of skin protectives o Preparation
of anticonvulsants o Preparation
of expectorants
STEP 7:
Evaluation (5 minutes)
What is Salt?
How do we categorize salt?
What are the characteristics of a salt?
What are the importance of salt in pharmacy?
STEP 8:
Assignment (10 hours)
|
Activity: Take home Assignment (5 minutes)
DIVIDE
students in groups or individual.
ASK the
students to work on the following assignment
Differentiate
acid, base and salt.
ALLOCATE time
for students to do the assignment and submit
REFER students to recommended references
|
References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6th ed.). New York, NY: The
McGraw-Hill Companies, Inc..
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rd Ed). London: Pharmaceutical press.
Manning, P. (2008). Essential
chemistry: Atoms, molecules and compounds. New York: Chelsea house.
Satyajit, D. S, & Lutfun, N. (2007). Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21st ed.). Baltimore, MA. A Wolters
Kluwer Company.
Session 21: Determination of pH and its Importance in pharmacy
Total Session Time: 60 minutes
Prerequisites
None
Learning Tasks
By the end of this session students are expected to be able
to:
Define
pH
Define
degree of dissociation
Explain the importance of degree of dissociation
Determine
pH of different acids
Describe
the use of pH scale
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
05 minutes
|
Presentation
|
Definition of pH
|
|
3
|
05 minutes
|
Presentation
|
Definition of Degree of
Dissociation
|
|
4
|
05 minutes
|
Presentation
|
Importance of Degree of
Dissociation
|
|
5
|
10 minutes
|
Presentation
|
Determination of pH of
Different Acid
|
|
6
|
10 minutes
|
Presentation
Brainstorming
|
Use of pH Scale
|
|
7
|
10 minutes
|
Presentation
|
Key Points
|
|
8
|
10 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Definition of pH (5 minutes)
pH
o
Refers to negative log (to base ten) of H+
ion concentration
o
pH
= 
pH
scale
o
Refers to a measure of the relative acidity or
alkalinity of a solution
STEP 3:
Definition of Degree of Dissociation (5 minutes)
Dissociation
o Refers
to the process by which compounds separate or split into smaller particles such
as atoms, ions or radicals
Degree
of dissociation o Refers
to the fraction of acid molecules that dissociate compared with the initial
concentration of the acid
o
The amount of solute dissociated into ions or
radicals per mole o Very strong acid and bases has a degree
of dissociation close to 1
o
Weak acids and bases have a degree of
dissociation less than that of the strong acids and bases
STEP 4:
Importance of Degree of Dissociation (5 minutes)
It
indicates the strength of acid o Using
the degree of dissociation of a particular acid/base it is easier to predict
its strength and thus help in studying the properties of pharmaceutical
ingredients such as solubility, stability, activity, and absorption
STEP 5:
Determination of pH of Different Acids (10 minutes)
Acidic
pH can be determined by the following methods:
o
Colorimetry
§ A
relatively simple and inexpensive method for determining the approximate pH of
a solution
§
Depends on the fact that some conjugate
acid-base pairs (indicators) possess one colour in the acid form and another
colour in the base form
§ Example
the acid form of a particular indicator is red, and the base form is yellow;
the colour of a solution of this indicator will range from red when it is
sufficiently acid, to yellow when it is sufficiently alkaline
o
Potentiometry
§ The
electrometric methods for the determination of pH
§ Base
on the difference of electrical potential between two suitable electrodes
dipping into a solution containing hydronium ions
§ Example
the pH meter is used in the laboratory to measure the pH of solutions Figure 21.1: A pH meter

Source: Chang General chemistry: The Essential Concepts (2011).
STEP 6: Use
of pH Scale (10 minutes)
|
Activity: Brainstorming (5 minutes)
Ask students to
brainstorm on the following question:
What
is the use of pH scale?
ALLOW few
students to respond
WRITE their
responses on the flip chart/ board
CLARIFY and SUMMARISE
by using the content below
|
The
pH scale is used for:
o
Studying the nature of pharmaceutical
ingredients by measuring the relative acidity or alkalinity
o
Maintenance of stability of pharmaceutical
dosage form by determining the pH of
various body organs example alkaline drugs are unstable in stomach pH and thus
a person is advised to take on empty stomach
o
Control of solubility of drugs
§ If
the drug is a weak acid or weak base, its solubility in water can be controlled
by the pH of the system
STEP 7: Key
Points (10 minutes)
pH
o
Refers to negative log (to base ten) of H+ ion
concentration
Degree
of dissociation o Refers
to the process by which compounds separate or split into smaller particles such
as atoms, ions or radicals
pH
can be determined by colorimetry and potentiometry method
Degree
of dissociation is important because it represents the strength of acid pH is important as
it is applied in:
o
Studying the nature of pharmaceutical
ingredients by measuring the relative acidity or alkalinity
o
Control of solubility of drugs
STEP 8:
Evaluation (10 minutes)
What
is pH?
What
is degree of Dissociation?
What
is the use of degree of dissociation?
What
is the use of pH?
References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6th ed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rd Ed). London: Pharmaceutical press.
Satyajit, D. S, & Lutfun, N. (2007). Chemistry for pharmacy students: General, Organic and Natural Product
Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21st ed.). Baltimore, MA. A Wolters
Kluwer Company.
Session 22: Description of Buffer Solutions and their Uses in Pharmacy
Total Session Time: 60 minutes
Prerequisites
None
Learning Tasks
By the end of this session students are expected to be able
to:
Define
buffer solutions and buffer capacity
List
Features of buffer solutions
Describe
uses of buffer solution
Resources Needed:
Flip
charts, marker pens, and masking tape
Black/white
board and chalk/whiteboard markers
SESSION OVERVIEW
|
Step
|
Time
|
Activity/
Method
|
Content
|
|
1
|
05 minutes
|
Presentation
|
Introduction, Learning
Tasks
|
|
2
|
10 minutes
|
Presentation
Brainstorming
|
Definition of Buffer Solution and Buffer
Capacity
|
|
3
|
20 minutes
|
Presentation
|
Features of Buffer
Solutions
|
|
4
|
10 minutes
|
Presentation
|
Uses of Buffer Solution
|
|
5
|
05 minutes
|
Presentation
|
Key Points
|
|
6
|
10 minutes
|
Presentation
|
Evaluation
|
SESSION CONTENTS
STEP 1:
Presentation of Session Title and Learning Tasks (5 minutes)
READ or ASK students
to read the learning tasks and clarify
ASK students if
they have any questions before continuing.
STEP 2:
Definition of Buffer Solution and Buffer Capacity (10 minutes)
|
Activity: Buzzing (5 minutes)
ASK students
to pair up and buzz on the following question for 2 minutes
What
is a buffer solution?
ALLOW few
pairs to respond and let other pairs to add on points not mentioned
WRITE their
response on the flip chart/board
CLARIFY and SUMMARIZE by
using the content below
|
Buffer
solution o Refers
to a solution that changes pH only slightly when small amounts of a strong acid
or a strong base is added
o
Contains a weak acid with its salt (conjugate
base) or a weak base with its salt
(conjugate acid) CH3COOH/CH3COONa
or NH3/NH4Cl respectively o The human body contains many buffer
systems, which control the pH of body compartments and fluids very effectively
o
Blood plasma is maintained at a pH of 7.4 by the
action of three main buffer systems:
§ Hydrogen
carbonate /carbonic acid buffer: Dissolved carbon dioxide, which gives carbonic
acid (H2CO3) in solution, and its sodium salt (usually
sodium bicarbonate, NaHCO3).It maintains the pH of the blood plasma
at a constant value of 7.35 7.45
§ Phosphate
buffer have an intracellular role Dihydrogenphosphate (H2PO4 -), with its
sodium salt
§ Protein
buffer o Therefore the human blood is a good
example of buffer solution
Buffer
capacity o The
ability to resist the change in pH after addition of a strong acid or strong
base. o The
amount of strong acid (e.g. HCl) or strong base (e.g. NaOH) in moles that can
be added to one litre of buffer in order to change the pH of the buffer by one
unit
o
If acid is added the pH does not fall
significantly; if base is added, the pH does not rise too
STEP 3:
Features of Buffer Solution (20 minutes)
Contains
a weak acid with its salt (conjugate base) or a weak base with its salt
(conjugate acid)
Resistant
to pH change in pH
Example
the protein buffer has the following features; o Proteins are polymers composed of
repeating units called amino acids containing
NH2 and COOH groups in the same molecule o Proteins are composed of about 20
different amino acids, which are connected to each other by peptide bonds
formed between one amino acid and its neighbour
o
The side-chain of the amino acid may be acidic
(as in the case of glutamic and aspartic acids), basic (as in the case of
arginine and lysine) or neutral (as in alanine)
o
Amino acids are amphoteric (capable of acting as
both acids and bases) o Most
proteins act as weak acids and form buffers with their sodium salts o These internal salts form zwitterion
(dipolar ion), and formation of the zwitterion makes the amino acid very polar
and therefore very soluble in water
o
If acid is added to the zwitterion, the ionised
COO- group will accept a proton to give un dissociated COOH and the overall
charge on the amino acid will be positive, due to the NH+
o
If base is added to the zwitterion, the NH3+
(which is really the conjugate acid of NH2) will function as an acid and donate
its proton to the base the overall charge on the amino acid will be negative,
due to the ionised COO-
o
Amino acids are ionised at all values of pH,
positively charged at low pH, negatively charged at high pH and zwitterionic at
neutral pH
STEP 4: Use
of Buffer Solution (10 minutes)
Maintain
the pH of a medicinal agent at an optimal value. o Bronsted acids and bases have been used
to maintain and adjust the pH of body fluids.
Improve solubility of drugs o Create
and maintain pH conditions in a system that cause the drug to be in its ionized
state
o
Ionized fraction of a drug is much more soluble
in water due to its increased polarity relative to the un-ionized fraction
STEP 5: Key
Points (5 minutes)
Buffer
solution o Refers
to a solution that changes pH only slightly when small amounts of a strong acid
or a strong base are added
Features
of Buffer solution include:
o
Contains a weak acid with its salt (conjugate
base) or a weak base with its salt
(conjugate acid) o Resistant to pH change in pH Buffer solutions are used to: o Maintain
the pH of a medicinal at an optimal value o Improve solubility
STEP 6:
Evaluation (10 minutes)
What
is buffer solution?
What
is buffer capacity?
What
are the features of buffer solutions?
What
are the uses of buffer solution?
References
Chang, R.,
& Overby, J. (2011). General
chemistry: The Essential Concepts (6th ed.). New York, NY: The
McGraw-Hill Companies, Inc.
Donald, C.
(2008). Essentials of Pharmaceutical
Chemistry (3rd Ed). London: Pharmaceutical press.
Satyajit, D. S., & Lutfun, N. (2007). Chemistry for pharmacy students: General,
Organic and Natural Product Chemistry. England: John Wiley & Sons Ltd.
Troy D. B.
(Ed.). (2005). Remington: The science and
practice of pharmacy. (21st ed.). Baltimore, MA. A Wolters
Kluwer Company.